Extracellular structure of polysialic acid explored by on cell solution NMR
- PMID: 17609375
- PMCID: PMC1906721
- DOI: 10.1073/pnas.0704404104
Extracellular structure of polysialic acid explored by on cell solution NMR
Abstract
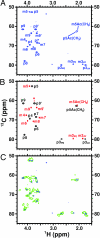
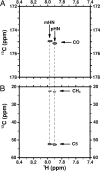
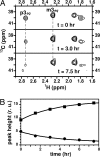
The capsular polysaccharide of the pathogens Neisseria meningitidis serogroup B and of Escherichia coli K1, alpha(2 --> 8) polysialic acid (PSA), is unusual, because when injected into adult humans, it generates little or no antibody. In contrast, people infected with these pathogens generate specific serum antibodies. A structural study on cells is used to address this anomaly by characterizing antigen structures in vivo. We introduce on cell multidimensional solution NMR spectroscopy for direct observation of PSA on E. coli bacteria. Using 13C,15N-labeled PSA, we applied a combination of heteronuclear NMR methods, such as heteronuclear single quantum coherence, HNCA, and HNCO, in vivo. Analysis reveals that free and cell-bound PSA are structurally similar, indicating that the poor immunogenicity of PSA is not due to major structural differences between cells and purified PSA. The 13C linewidths of PSA on cells are 2 to 3 times larger than the corresponding ones in free PSA. The possible implications of the differences between free and on cell PSA are discussed. In addition, we demonstrate the suitability of the method for in vivo kinetic studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Robbins JB, Schneerson R, Anderson P, Smith DH. J Am Med Assoc. 1996;276:1181–1186. - PubMed
-
- Robbins JB, McCracken GH, Gotschlich EC, Ørskov F, Ørskov I, Hanson LA. N Engl J Med. 1974;290:12216–12220. - PubMed
-
- Bhattacharjee AK, Jennings HJ, Kenny CP, Martin A, Smith IC. J Biol Chem. 1975;250:1926–1932. - PubMed
-
- Wyle FA, Artenstein MS, Brandt BL, Tramont EC, Kasper DL, Altieri PL, Berman SL, Lowenthal JP. J Infect Dis. 1972;126:514–522. - PubMed
-
- Finne J, Leinonen M, Mäkelä P. H. The Lancet. 1983;2:355–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

