Structural consequences of disease-causing mutations in the ATRX-DNMT3-DNMT3L (ADD) domain of the chromatin-associated protein ATRX
- PMID: 17609377
- PMCID: PMC1924575
- DOI: 10.1073/pnas.0704057104
Structural consequences of disease-causing mutations in the ATRX-DNMT3-DNMT3L (ADD) domain of the chromatin-associated protein ATRX
Abstract
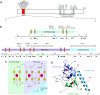
The chromatin-associated protein ATRX was originally identified because mutations in the ATRX gene cause a severe form of syndromal X-linked mental retardation associated with alpha-thalassemia. Half of all of the disease-associated missense mutations cluster in a cysteine-rich region in the N terminus of ATRX. This region was named the ATRX-DNMT3-DNMT3L (ADD) domain, based on sequence homology with a family of DNA methyltransferases. Here, we report the solution structure of the ADD domain of ATRX, which consists of an N-terminal GATA-like zinc finger, a plant homeodomain finger, and a long C-terminal alpha-helix that pack together to form a single globular domain. Interestingly, the alpha-helix of the GATA-like finger is exposed and highly basic, suggesting a DNA-binding function for ATRX. The disease-causing mutations fall into two groups: the majority affect buried residues and hence affect the structural integrity of the ADD domain; another group affects a cluster of surface residues, and these are likely to perturb a potential protein interaction site. The effects of individual point mutations on the folding state and stability of the ADD domain correlate well with the levels of mutant ATRX protein in patients, providing insights into the molecular pathophysiology of ATR-X syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
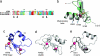


References
-
- Weatherall DJ, Higgs DR, Bunch C, Old JM, Hunt DM, Pressley L, Clegg JB, Bethlenfalvay NC, Sjolin S, Koler RD, et al. N Engl J Med. 1981;305:607–612. - PubMed
-
- Gibbons RJ, Picketts DJ, Villard L, Higgs DR. Cell. 1995;80:837–845. - PubMed
-
- Gibbons RJ, Pellagatti A, Garrick D, Wood WG, Malik N, Ayyub H, Langford C, Boultwood J, Wainscoat JS, Higgs DR. Nat Genet. 2003;34:446–449. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

