PIP5KI gamma is required for cardiovascular and neuronal development
- PMID: 17609388
- PMCID: PMC1913884
- DOI: 10.1073/pnas.0700019104
PIP5KI gamma is required for cardiovascular and neuronal development
Abstract
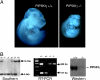
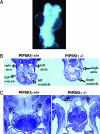
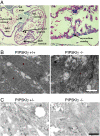
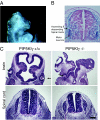
All eukaryotic cells contain the phospholipid phosphatidylinositol 4, 5-bisphosphate (PIP2) that serves multiple roles in signal transduction cascades. Type I phosphatidylinositol-4-phosphate 5-kinase (PIP5KI) catalyzes the synthesis of PIP2 by phosphorylating phosphatidylinositol 4 phosphate. Although the classical isoforms of PIP5KI (designated as alpha, beta, and gamma) all generate the same phospholipid product, they have significantly dissimilar primary structures and expression levels in different tissues, and they appear to localize within different compartments within the cell. Therefore, it appears likely that PIP5KI isoforms have overlapping, but not identical, functions. Here we show that targeted disruption of PIP5KIgamma causes widespread developmental and cellular defects. PIP5KIgamma-null embryos have myocardial developmental defects associated with impaired intracellular junctions that lead to heart failure and extensive prenatal lethality at embryonic day 11.5 of development. Loss of PIP5KIgamma also results in neural tube closure defects that were associated with impaired PIP2 production, adhesion junction formation, and neuronal cell migration. These data, along with those of other PIP5KI isoforms, indicate that individual PIP5KI isoenzymes fulfill specific roles in embryonic development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hokin MR, Hokin LE. J Biol Chem. 1953;203:967–977. - PubMed
-
- Toker A. Curr Opin Cell Biol. 1998;10:254–261. - PubMed
-
- Doughman RL, Firestone AJ, Anderson RA. J Membr Biol. 2003;194:77–89. - PubMed
-
- Ishihara H, Shibasaki Y, Kizuki N, Katagiri H, Yazaki Y, Asano T, Oka Y. J Biol Chem. 1996;271:23611–23614. - PubMed
-
- Loijens JC, Anderson RA. J Biol Chem. 1996;271:32937–32943. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

