Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver
- PMID: 17609423
- PMCID: PMC1988950
- DOI: 10.1182/blood-2007-03-080093
Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver
Abstract
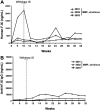
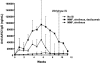
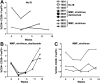
Adeno-associated virus (AAV)-mediated gene transfer of factor IX (F.IX) to the liver results in long-term expression of transgene in experimental animals, but only short-term expression in humans. Loss of F.IX expression is likely due to a cytotoxic immune response to the AAV capsid, which results in clearance of transduced hepatocytes. We used a nonhuman primate model to assess the safety of AAV gene transfer coupled with an anti-T-cell regimen designed to block this immune response. Administration of a 3-drug regimen consisting of mycophenolate mofetil (MMF), sirolimus, and the anti-IL-2 receptor antibody daclizumab consistently resulted in formation of inhibitory antibodies to human F.IX following hepatic artery administration of an AAV-hF.IX vector, whereas a 2-drug regimen consisting only of MMF and sirolimus did not. Administration of daclizumab was accompanied by a dramatic drop in the population of CD4(+)CD25(+)FoxP3(+) regulatory T cells (Tregs). We conclude that choice of immunosuppression (IS) regimen can modulate immune responses to the transgene product upon hepatic gene transfer in subjects not fully tolerant; and that induction of transgene tolerance may depend on a population of antigen-specific Tregs.
Figures

References
-
- Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. - PubMed
-
- Cardone M, Polito VA, Pepe S, et al. Correction of Hunter syndrome in the MPSII mouse model by AAV2/8-mediated gene delivery. Hum Mol Genet. 2006;15:1225–1236. - PubMed
-
- Daly TM, Ohlemiller KK, Roberts MS, Vogler CA, Sands MS. Prevention of systemic clinical disease in MPS VII mice following AAV-mediated neonatal gene transfer. Gene Ther. 2001;8:1291–1298. - PubMed
-
- McEachern KA, Nietupski JB, Chuang WL, et al. AAV8-mediated expression of glucocerebrosidase ameliorates the storage pathology in the visceral organs of a mouse model of Gaucher disease. J Gene Med. 2006;8:719–729. - PubMed
-
- Koeberl DD, Sun BD, Damodaran TV, et al. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 2006;13:1281–1289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

