Salinosporamide A (NPI-0052) potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through down-modulation of NF-kappaB regulated gene products
- PMID: 17609425
- PMCID: PMC1988928
- DOI: 10.1182/blood-2007-04-084996
Salinosporamide A (NPI-0052) potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through down-modulation of NF-kappaB regulated gene products
Abstract
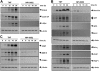
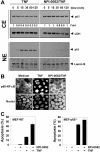
Salinosporamide A (also called NPI-0052), recently identified from the marine bacterium Salinispora tropica, is a potent inhibitor of 20S proteasome and exhibits therapeutic potential against a wide variety of tumors through a poorly understood mechanism. Here we demonstrate that salinosporamide A potentiated the apoptosis induced by tumor necrosis factor alpha (TNF), bortezomib, and thalidomide, and this correlated with down-regulation of gene products that mediate cell proliferation (cyclin D1, cyclooxygenase-2 [COX-2], and c-Myc), cell survival (Bcl-2, Bcl-xL, cFLIP, TRAF1, IAP1, IAP2, and survivin), invasion (matrix metallopro-teinase-9 [MMP-9] and ICAM-1), and angiogenesis (vascular endothelial growth factor [VEGF]). Salinosporamide A also suppressed TNF-induced tumor cell invasion and receptor activator of nuclear factor kappaB ligand (RANKL)-induced osteoclastogenesis. We also found that it suppressed both constitutive and inducible NF-kappaB activation. Compared with bortezomib, MG-132, N-acetyl-leucyl-leucyl-norleucinal (ALLN), and lactacystin, salinosporamide A was found to be the most potent suppressor of NF-kappaB activation. Further studies showed that salinosporamide A inhibited TNF-induced inhibitory subunit of NF-kappaB alpha (IkappaBalpha) degradation, nuclear translocation of p65, and NF-kappaB-dependent reporter gene expression but had no effect on IkappaBalpha kinase activation, IkappaBalpha phosphorylation, or IkappaBalpha ubiquitination. Thus, overall, our results indicate that salinosporamide A enhances apoptosis, suppresses osteoclastogenesis, and inhibits invasion through suppression of the NF-kappaB pathway.
Figures





References
-
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–1037. - PubMed
-
- Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed Engl. 2003;42:355–357. - PubMed
-
- Jensen PR, Mincer TJ, Williams PG, Fenical W. Marine actinomycete diversity and natural product discovery. Antonie Van Leeuwenhoek. 2005;87:43–48. - PubMed
-
- Macherla VR, Mitchell SS, Manam RR, et al. Structure-activity relationship studies of Salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J Med Chem. 2005;48:3684–3687. - PubMed
-
- Groll M, Huber R, Potts BC. Crystal structures of Salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. J Am Chem Soc. 2006;128:5136–5141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

