Reviewing Chandipura: a vesiculovirus in human epidemics
- PMID: 17610154
- PMCID: PMC7087735
- DOI: 10.1007/s10540-007-9054-z
Reviewing Chandipura: a vesiculovirus in human epidemics
Abstract
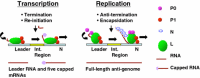
Chandipura virus, a member of the rhabdoviridae family and vesiculovirus genera, has recently emerged as human pathogen that is associated with a number of outbreaks in different parts of India. Although, the virus closely resembles with the prototype vesiculovirus, Vesicular Stomatitis Virus, it could be readily distinguished by its ability to infect humans. Studies on Chandipura virus while shed light into distinct stages of viral infection; it may also allow us to identify potential drug targets for antiviral therapy. In this review, we have summarized our current understanding of Chandipura virus life cycle at the molecular detail with particular interest in viral RNA metabolisms, namely transcription, replication and packaging of viral RNA into nucleocapsid structure. Contemporary research on otherwise extensively studied family member Vesicular Stomatitis Virus has also been addressed to present a more comprehensive picture of vesiculovirus life cycle. Finally, we reveal examples of protein economy in Chandipura virus life-cycle whereby each viral protein has evolved complexity to perform multiple tasks.
Figures

References
-
- Ahlquist P. RNA-dependent RNA polymerases, viruses, and RNA silencing. Science. 2002;296:1270–1273. - PubMed
-
- Albertini AA, Wernimont AK, Muziol T, Ravelli RB, Clapier CR, Schoehn G, Weissenhorn W, Ruigrok RW. Crystal structure of the rabies virus Nucleoprotein-RNA complex. Science. 2006;313:360–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

