Atomoxetine pharmacokinetics in healthy Chinese subjects and effect of the CYP2D6*10 allele
- PMID: 17610534
- PMCID: PMC2048549
- DOI: 10.1111/j.1365-2125.2007.02912.x
Atomoxetine pharmacokinetics in healthy Chinese subjects and effect of the CYP2D6*10 allele
Abstract
Aims: To characterize atomoxetine pharmacokinetics, explore the effect of the homozygous CYP2D6*10 genotype on atomoxetine pharmacokinetics and evaluate the tolerability of atomoxetine, in healthy Chinese subjects.
Methods: Twenty-four subjects, all CYP2D6 extensive metabolizers (EM), were randomized to receive atomoxetine (40 mg qd for 3 days, then 80 mg qd for 7 days) or matching placebo (2 : 1 ratio) in a double-blind fashion. Atomoxetine serum concentrations were measured following single (40 mg) and multiple (80 mg) doses. Adverse events, clinical safety laboratory data and vital signs were assessed during the study.
Results: Atomoxetine was rapidly absorbed with median time to maximum serum concentrations of approximately 1.5 h after single and multiple doses. Atomoxetine concentrations appeared to decrease monoexponentially with a mean apparent terminal half-life (t(1/2)) of approximately 4 h. The apparent clearance, apparent volume of distribution and t(1/2) following single and multiple doses were similar, suggesting linear pharmacokinetics with respect to time. Homozygous CYP2D6*10 subjects had 50% lower clearances compared with other EM subjects, resulting in twofold higher mean exposures. No clinically significant changes or abnormalities were noted in laboratory data and vital signs.
Conclusions: The pharmacokinetics of atomoxetine in healthy Chinese subjects appears comparable to other ethnic populations. Multiple dosing of 80 mg qd atomoxetine was well tolerated in this study.
Figures
References
-
- Michelson D, Adler L, Spencer T, Reimherr FW, West SA, Allen AJ, Kelsey D, Wernicke J, Dietrich A, Milton D. Atomoxetine in adults with attention-deficit/hyperactivity disorder: two randomised, placebo-controlled studies. Biol Psychiatry. 2003;53:112–20. - PubMed
-
- Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, Newcorn J, Sallee FR, Sangal RB, Saylor K, West S, Kelsey D, Wernicke J, Trapp NJ, Harder D. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomised, placebo-controlled study. Am J Psychiatry. 2002;159:1896–901. - PubMed
-
- Ring BJ, Gillespie JS, Eckstein JA, Wrighton SA. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30:319–23. - PubMed
-
- Sauer JM, Ponsler GD, Mattiuz EL, Long AJ, Witcher JW, Thomasson HR, Desante KA. Disposition and metabolic fate of atomoxetine hydrochloride. The role of CYP2D6 in human disposition and metabolism. Drug Metab Dispos. 2003;31:98–107. - PubMed
-
- Guengerich FP. Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism and Biochemistry. New York: Plenum Press; 1995. pp. 473–535.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

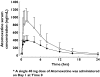
 ) and on day 10 following multiple doses of 80 mg qd (N = 16) (○)
) and on day 10 following multiple doses of 80 mg qd (N = 16) (○)