Phenotypic alterations in type II alveolar epithelial cells in CD4+ T cell mediated lung inflammation
- PMID: 17610738
- PMCID: PMC1939847
- DOI: 10.1186/1465-9921-8-47
Phenotypic alterations in type II alveolar epithelial cells in CD4+ T cell mediated lung inflammation
Abstract
Background: Although the contribution of alveolar type II epithelial cell (AEC II) activities in various aspects of respiratory immune regulation has become increasingly appreciated, our understanding of the contribution of AEC II transcriptosome in immunopathologic lung injury remains poorly understood. We have previously established a mouse model for chronic T cell-mediated pulmonary inflammation in which influenza hemagglutinin (HA) is expressed as a transgene in AEC II, in mice expressing a transgenic T cell receptor specific for a class II-restricted epitope of HA. Pulmonary inflammation in these mice occurs as a result of CD4+ T cell recognition of alveolar antigen. This model was utilized to assess the profile of inflammatory mediators expressed by alveolar epithelial target cells triggered by antigen-specific recognition in CD4+ T cell-mediated lung inflammation.
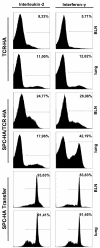
Methods: We established a method that allows the flow cytometric negative selection and isolation of primary AEC II of high viability and purity. Genome wide transcriptional profiling was performed on mRNA isolated from AEC II isolated from healthy mice and from mice with acute and chronic CD4+ T cell-mediated pulmonary inflammation.
Results: T cell-mediated inflammation was associated with expression of a broad array of cytokine and chemokine genes by AEC II cell, indicating a potential contribution of epithelial-derived chemoattractants to the inflammatory cell parenchymal infiltration. Morphologically, there was an increase in the size of activated epithelial cells, and on the molecular level, comparative transcriptome analyses of AEC II from inflamed versus normal lungs provide a detailed characterization of the specific inflammatory genes expressed in AEC II induced in the context of CD4+ T cell-mediated pneumonitis.
Conclusion: An important contribution of AEC II gene expression to the orchestration and regulation of interstitial pneumonitis is suggested by the panoply of inflammatory genes expressed by this cell population, and this may provide insight into the molecular pathogenesis of pulmonary inflammatory states. CD4+ T cell recognition of antigen presented by AEC II cells appears to be a potent trigger for activation of the alveolar cell inflammatory transcriptosome.
Figures





References
-
- Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–337. - PubMed
-
- Kalina M, Mason RJ, Shannon JM. Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am J Respir Cell Mol Biol. 1992;6:594–600. - PubMed
-
- Lesur O, Arsalane K, Lane D. Lung alveolar epithelial cell migration in vitro: modulators and regulation processes. Am J Physiol. 1996;270:L311–L319. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

