Recombinant production of Streptococcus equisimilis streptokinase by Streptomyces lividans
- PMID: 17610745
- PMCID: PMC1936427
- DOI: 10.1186/1475-2859-6-20
Recombinant production of Streptococcus equisimilis streptokinase by Streptomyces lividans
Abstract
Background: Streptokinase (SK) is a potent plasminogen activator with widespread clinical use as a thrombolytic agent. It is naturally secreted by several strains of beta-haemolytic streptococci. The low yields obtained in SK production, lack of developed gene transfer methodology and the pathogenesis of its natural host have been the principal reasons to search for a recombinant source for this important therapeutic protein. We report here the expression and secretion of SK by the Gram-positive bacterium Streptomyces lividans. The structural gene encoding SK was fused to the Streptomyces venezuelae CBS762.70 subtilisin inhibitor (vsi) signal sequence or to the Streptomyces lividans xylanase C (xlnC) signal sequence. The native Vsi protein is translocated via the Sec pathway while the native XlnC protein uses the twin-arginine translocation (Tat) pathway.
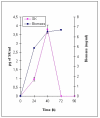
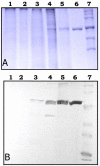
Results: SK yield in the spent culture medium of S. lividans was higher when the Sec-dependent signal peptide mediates the SK translocation. Using a 1.5 L fermentor, the secretory production of the Vsi-SK fusion protein reached up to 15 mg SK/l. SK was partially purified from the culture supernatant by DEAE-Sephacel chromatography. A 44-kDa degradation product co-eluted with the 47-kDa mature SK. The first amino acid residues of the S. lividans-produced SK were identical with those of the expected N-terminal sequence. The Vsi signal peptide was thus correctly cleaved off and the N-terminus of mature Vsi-SK fusion protein released by S. lividans remained intact. This result also implicates that the processing of the recombinant SK secreted by Streptomyces probably occurred at its C-terminal end, as in its native host Streptococcus equisimilis. The specific activity of the partially purified Streptomyces-derived SK was determined at 2661 IU/mg protein.
Conclusion: Heterologous expression of Streptococcus equisimilis ATCC9542 skc-2 in Streptomyces lividans was successfully achieved. SK can be translocated via both the Sec and the Tat pathway in S. lividans, but yield was about 30 times higher when the SK was fused to the Sec-dependent Vsi signal peptide compared to the fusion with the Tat-dependent signal peptide of S. lividans xylanase C. Small-scale fermentation led to a fourfold improvement of secretory SK yield in S. lividans compared to lab-scale conditions. The partially purified SK showed biological activity. Streptomyces lividans was shown to be a valuable host for the production of a world-wide important, biopharmaceutical product in a bio-active form.
Figures

Similar articles
-
Use of Strep-tag II for rapid detection and purification of Mycobacterium tuberculosis recombinant antigens secreted by Streptomyces lividans.J Microbiol Methods. 2013 Sep;94(3):192-8. doi: 10.1016/j.mimet.2013.06.004. Epub 2013 Jun 17. J Microbiol Methods. 2013. PMID: 23791917
-
Comparison of the Sec and Tat secretion pathways for heterologous protein production by Streptomyces lividans.J Biotechnol. 2004 Sep 9;112(3):279-88. doi: 10.1016/j.jbiotec.2004.05.004. J Biotechnol. 2004. PMID: 15313005
-
Secretion of active xylanase C from Streptomyces lividans is exclusively mediated by the Tat protein export system.Biochim Biophys Acta. 2004 Jun 1;1699(1-2):155-62. doi: 10.1016/j.bbapap.2004.02.008. Biochim Biophys Acta. 2004. PMID: 15158723
-
Streptomyces protein secretion and its application in biotechnology.FEMS Microbiol Lett. 2018 Nov 1;365(22). doi: 10.1093/femsle/fny250. FEMS Microbiol Lett. 2018. PMID: 30299471 Review.
-
Protein secretion biotechnology in Gram-positive bacteria with special emphasis on Streptomyces lividans.Biochim Biophys Acta. 2014 Aug;1843(8):1750-61. doi: 10.1016/j.bbamcr.2013.12.023. Epub 2014 Jan 9. Biochim Biophys Acta. 2014. PMID: 24412306 Review.
Cited by
-
CRISPR-Cas9 Mediated Knockout of SagD Gene for Overexpression of Streptokinase in Streptococcus equisimilis.Microorganisms. 2022 Mar 17;10(3):635. doi: 10.3390/microorganisms10030635. Microorganisms. 2022. PMID: 35336210 Free PMC article.
-
Penicillium citrinum CFAM 521 Isolated From the Amazon Region: A Novel Source of a Fibrinolytic Enzyme.Int J Microbiol. 2024 Oct 29;2024:5306083. doi: 10.1155/2024/5306083. eCollection 2024. Int J Microbiol. 2024. PMID: 39502513 Free PMC article.
-
Streptomycetes: Attractive Hosts for Recombinant Protein Production.Front Microbiol. 2020 Aug 20;11:1958. doi: 10.3389/fmicb.2020.01958. eCollection 2020. Front Microbiol. 2020. PMID: 32973711 Free PMC article. Review.
-
The O-mannosylation and production of recombinant APA (45/47 KDa) protein from Mycobacterium tuberculosis in Streptomyces lividans is affected by culture conditions in shake flasks.Microb Cell Fact. 2011 Dec 20;10:110. doi: 10.1186/1475-2859-10-110. Microb Cell Fact. 2011. PMID: 22185589 Free PMC article.
-
Highly effective renaturation of a streptokinase from Streptococcus pyogenes DT7 as inclusion bodies overexpressed in Escherichia coli.Biomed Res Int. 2014;2014:324705. doi: 10.1155/2014/324705. Epub 2014 May 5. Biomed Res Int. 2014. PMID: 24883307 Free PMC article.
References
-
- Sherry S, Fletcher AP, Alkjaersig N. Fibrinolysis and fibrinolytic activity in man. Physiol Rev. 1959;39:343–382. - PubMed
-
- Zhang XW, Sun T, Huang XN, Liu X, Gu DX, Tang ZQ. Recombinant streptokinase production by fed-batch cultivation of Escherichia coli. Enzyme Microb Technol. 1999;24:647–650. doi: 10.1016/S0141-0229(98)00149-5. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

