Standing waves and traveling waves distinguish two circuits in visual cortex
- PMID: 17610820
- PMCID: PMC2171365
- DOI: 10.1016/j.neuron.2007.06.017
Standing waves and traveling waves distinguish two circuits in visual cortex
Abstract
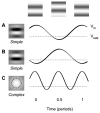
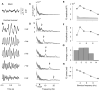
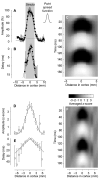
The visual cortex represents stimuli through the activity of neuronal populations. We measured the evolution of this activity in space and time by imaging voltage-sensitive dyes in cat area V1. Contrast-reversing stimuli elicit responses that oscillate at twice the stimulus frequency, indicating that signals originate mostly in complex cells. These responses stand clear of the noise, whose amplitude decreases as 1/frequency, and yield high-resolution maps of orientation preference and retinotopy. We first show how these maps are combined to yield the responses to focal, oriented stimuli. We then study the evolution of the oscillating activity in space and time. In the orientation domain, it is a standing wave. In the spatial domain, it is a traveling wave propagating at 0.2-0.5 m/s. These different dynamics indicate a fundamental distinction in the circuits underlying selectivity for position and orientation, two key stimulus attributes.
Figures


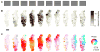

Comment in
-
Propagating waves in visual cortex.Neuron. 2007 Jul 5;55(1):3-5. doi: 10.1016/j.neuron.2007.06.027. Neuron. 2007. PMID: 17610811
References
-
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. I. The precision of the topography. Exp Brain Res. 1975;24:159–179. - PubMed
-
- Anderson J, Carandini M, Ferster D. Orientation tuning of input conductance, excitation and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

