Translational control of meiotic cell cycle progression and spermatid differentiation in male germ cells by a novel eIF4G homolog
- PMID: 17611220
- PMCID: PMC4620998
- DOI: 10.1242/dev.003764
Translational control of meiotic cell cycle progression and spermatid differentiation in male germ cells by a novel eIF4G homolog
Abstract
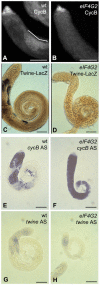
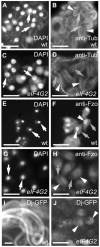
Translational control is crucial for proper timing of developmental events that take place in the absence of transcription, as in meiotic activation in oocytes, early embryogenesis in many organisms, and spermatogenesis. Here we show that a novel form of the translation initiation complex component eIF4G in Drosophila, eIF4G2, is required specifically for male germ cells to undergo meiotic division and proper spermatid differentiation. Flies mutant for eIF4G2 are viable and female fertile but male sterile. Spermatocytes form, but the germ cells in mutant males skip the major events of the meiotic divisions and form aberrant spermatids with large nuclei. Consistent with the failure to undergo the meiotic divisions, function of eIF4G2 is required post-transcriptionally for normal accumulation of the core cell cycle regulatory proteins Twine and CycB in mature spermatocytes. Loss of eIF4G2 function also causes widespread defects in spermatid differentiation. Although differentiation markers Dj and Fzo are expressed in late-stage eIF4G2 mutant germ cells, several key steps of spermatid differentiation fail, including formation of a compact mitochondrial derivative and full elongation. Our results suggest that an alternate form of the translation initiation machinery may be required for regulation and execution of key steps in male germ cell differentiation.
Figures





References
-
- Alphey L, Jimenez J, White-Cooper H, Dawson I, Nurse P, Glover DM. Twine, a CDC25 homolog that functions in the male and female germline of Drosophila. Cell. 1992;69:977–988. - PubMed
-
- Chen X, Fischer JA. A P element transformation vector for high levels of gene expression in germ-line cells of the ovary and undifferentiated cells in the developing eye of Drosophila. Plasmid. 2002;47:61–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

