Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation
- PMID: 17611263
- PMCID: PMC6794577
- DOI: 10.1523/JNEUROSCI.0264-07.2007
Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation
Abstract
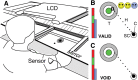
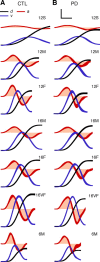
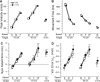
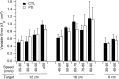
People generally select a similar speed for a given motor task, such as reaching for a cup. One well established determinant of movement time is the speed-accuracy trade-off: movement time increases with the accuracy requirement. A second possible determinant is the energetic cost of making a movement. Parkinson's disease (PD), a condition characterized by generalized movement slowing (bradykinesia), provides the opportunity to directly explore this second possibility. We compared reaching movements of patients with PD with those of control subjects in a speed-accuracy trade-off task comprising conditions of increasing difficulty. Subjects completed as many trials as necessary to make 20 movements within a required speed range (trials to criterion, N(c)). Difficulty was reflected in endpoint accuracy and N(c). Patients were as accurate as control subjects in all conditions (i.e., PD did not affect the speed-accuracy trade-off). However, N(c) was consistently higher in patients, indicating reluctance to move fast although accuracy was not compromised. Specifically, the dependence of N(c) on movement energy cost (slope S(N)) was steeper in patients than in control subjects. This difference in S(N) suggests that bradykinesia represents an implicit decision not to move fast because of a shift in the cost/benefit ratio of the energy expenditure needed to move at normal speed. S(N) was less steep, but statistically significant, in control subjects, which demonstrates a role for energetic cost in the normal control of movement speed. We propose that, analogous to the established role of dopamine in explicit reward-seeking behavior, the dopaminergic projection to the striatum provides a signal for implicit "motor motivation."
Figures

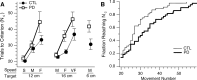

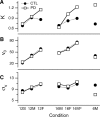
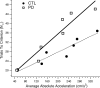
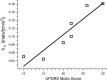
Comment in
-
Parkinson's disease: fighting the will?J Neurosci. 2007 Oct 31;27(44):11777-9. doi: 10.1523/JNEUROSCI.4010-07.2007. J Neurosci. 2007. PMID: 17978012 Free PMC article. No abstract available.
References
-
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001;124:2131–2146. - PubMed
-
- Brumlik J, Boshes B. The mechanism of bradykinesia in parkinsonism. Neurology. 1966;16:337–344. - PubMed
-
- England AC, Schwab RS. Parkinson's syndrome. N Engl J Med. 1961;265:785–792. - PubMed
-
- Evarts EV, Teravainen H, Calne DB. Reaction time in Parkinson's disease. Brain. 1981;104:167–186. - PubMed
-
- Fahn S, Elton RL. the members of the UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson's disease II. New York: MacMillan; 1987. pp. 153–163.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
