Gene cluster lock after pheromone receptor gene choice
- PMID: 17611603
- PMCID: PMC1933412
- DOI: 10.1038/sj.emboj.7601782
Gene cluster lock after pheromone receptor gene choice
Abstract
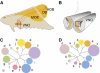
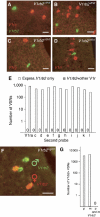
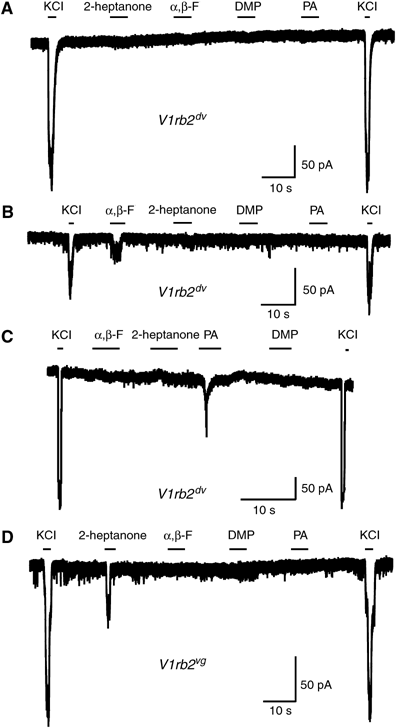
In mammals, perception of pheromones is based on the expression in each vomeronasal sensory neuron of a limited set of receptor genes, chosen among a large repertoire. Here, we report an extremely tight control of the monogenic and monoallelic transcription of the V1rb2 receptor gene. Combining genetic and electrophysiological approaches, we show that the transcription of a non-functional V1r allele leads to the coexpression of another, functional V1r gene. The choice of this coexpressed gene surprisingly includes genes located on the cluster homologous to the one from which the mutant allele is transcribed. However, V1r genes located in cis relative to the transcribed mutant allele are excluded from the coexpression choice. Our observations strongly suggest a monogenic regulatory mechanism acting (a) at a general level, via the expression of the V1r receptor itself, and (b) at a more local level, defined by the V1r gene cluster.
Figures


Similar articles
-
Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes.Nature. 2002 Sep 5;419(6902):70-4. doi: 10.1038/nature00955. Nature. 2002. PMID: 12214233
-
Olfactory expression of a single and highly variable V1r pheromone receptor-like gene in fish species.Proc Natl Acad Sci U S A. 2005 Apr 12;102(15):5489-94. doi: 10.1073/pnas.0402581102. Epub 2005 Apr 4. Proc Natl Acad Sci U S A. 2005. PMID: 15809442 Free PMC article.
-
Rapid turnover and species-specificity of vomeronasal pheromone receptor genes in mice and rats.Gene. 2004 Oct 13;340(2):303-12. doi: 10.1016/j.gene.2004.07.037. Gene. 2004. PMID: 15475172
-
Pheromone receptors in mammals.Horm Behav. 2004 Sep;46(3):219-30. doi: 10.1016/j.yhbeh.2004.03.014. Horm Behav. 2004. PMID: 15325223 Review.
-
Odorant and pheromone receptor gene regulation in vertebrates.Curr Opin Genet Dev. 2007 Oct;17(5):465-70. doi: 10.1016/j.gde.2007.07.005. Epub 2007 Aug 20. Curr Opin Genet Dev. 2007. PMID: 17709237 Review.
Cited by
-
A purely bioinformatic pipeline for the prediction of mammalian odorant receptor gene enhancers.BMC Bioinformatics. 2019 Sep 14;20(1):474. doi: 10.1186/s12859-019-3012-1. BMC Bioinformatics. 2019. PMID: 31521109 Free PMC article.
-
Functional promiscuity in a mammalian chemosensory system: extensive expression of vomeronasal receptors in the main olfactory epithelium of mouse lemurs.Front Neuroanat. 2014 Sep 24;8:102. doi: 10.3389/fnana.2014.00102. eCollection 2014. Front Neuroanat. 2014. PMID: 25309343 Free PMC article.
-
Mice with a "monoclonal nose": perturbations in an olfactory map impair odor discrimination.Neuron. 2008 Dec 26;60(6):1068-81. doi: 10.1016/j.neuron.2008.10.046. Neuron. 2008. PMID: 19109912 Free PMC article.
-
Rescue of an aggressive female sexual courtship in mice by CRISPR/Cas9 secondary mutation in vivo.BMC Res Notes. 2018 Mar 27;11(1):193. doi: 10.1186/s13104-018-3307-8. BMC Res Notes. 2018. PMID: 29580290 Free PMC article.
-
Molecular, Cellular, and Developmental Organization of the Mouse Vomeronasal organ at Single Cell Resolution.bioRxiv [Preprint]. 2024 Sep 5:2024.02.22.581574. doi: 10.1101/2024.02.22.581574. bioRxiv. 2024. Update in: Elife. 2024 Dec 10;13:RP97356. doi: 10.7554/eLife.97356. PMID: 39253476 Free PMC article. Updated. Preprint.
References
-
- Belluscio L, Koentges G, Axel R, Dulac C (1999) A map of pheromone receptor activation in the mammalian brain. Cell 97: 209–220 - PubMed
-
- Boschat C, Pelofi C, Randin O, Roppolo D, Luscher C, Broillet MC, Rodriguez I (2002) Pheromone detection mediated by a V1r vomeronasal receptor. Nat Neurosci 5: 1261–1262 - PubMed
-
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P (2002) Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419: 70–74 - PubMed
-
- Dulac C, Axel R (1995) A novel family of genes encoding putative pheromone receptors in mammals. Cell 83: 195–206 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

