Immunodominance of lytic cycle antigens in Epstein-Barr virus-specific CD4+ T cell preparations for therapy
- PMID: 17611619
- PMCID: PMC1894652
- DOI: 10.1371/journal.pone.0000583
Immunodominance of lytic cycle antigens in Epstein-Barr virus-specific CD4+ T cell preparations for therapy
Abstract
Background: Epstein-Barr virus (EBV) is associated with a number of human malignancies. EBV-positive post-transplant lymphoproliferative disease in solid organ and hematopoietic stem cell transplant recipients has been successfully treated by the adoptive transfer of polyclonal EBV-specific T cell lines containing CD4+ and CD8+ T cell components. Although patients receiving T cell preparations with a higher CD4+ T cell proportion show better clinical responses, the specificity of the infused CD4+ component has remained completely unknown.
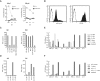
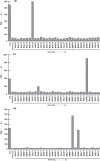
Methodology/principal findings: We generated LCL-stimulated T cell lines from 21 donors according to clinical protocols, and analyzed the antigen specificity of the CD4+ component in EBV-specific T cell preparations using a genetically engineered EBV mutant that is unable to enter the lytic cycle, and recombinantly expressed and purified EBV proteins. Surprisingly, CD4+ T cell lines from acutely and persistently EBV-infected donors consistently responded against EBV lytic cycle antigens and autoantigens, but barely against latent cycle antigens of EBV hitherto considered principal immunotherapeutic targets. Lytic cycle antigens were predominantly derived from structural proteins of the virus presented on MHC II via receptor-mediated uptake of released viral particles, but also included abundant infected cell proteins whose presentation involved intercellular protein transfer. Importantly, presentation of virion antigens was severely impaired by acyclovir treatment of stimulator cells, as currently performed in most clinical protocols.
Conclusions/significance: These results indicate that structural antigens of EBV are the immunodominant targets of CD4+ T cells in LCL-stimulated T cell preparations. These findings add to our understanding of the immune response against this human tumor-virus and have important implications for the improvement of immunotherapeutic strategies against EBV.
Conflict of interest statement
Figures
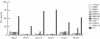
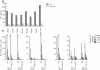

References
-
- Kuppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3:801–812. - PubMed
-
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. - PubMed
-
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Field's Virology. 5th ed. Philadelphia: Lippincott-Raven; 2006. pp. 2655–2700.
-
- Papesch M, Watkins R. Epstein-Barr virus infectious mononucleosis. Clin Otolaryngol Allied Sci. 2001;26:3–8. - PubMed
-
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

