Mitochondria and Ca(2+) signaling: old guests, new functions
- PMID: 17611770
- PMCID: PMC4864527
- DOI: 10.1007/s00424-007-0296-1
Mitochondria and Ca(2+) signaling: old guests, new functions
Abstract
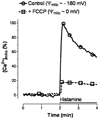
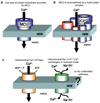
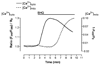
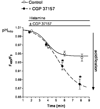
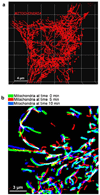
Mitochondria are ancient endosymbiotic guests that joined the cells in the evolution of complex life. While the unique ability of mitochondria to produce adenosine triphosphate (ATP) and their contribution to cellular nutrition metabolism received condign attention, our understanding of the organelle's contribution to Ca(2+) homeostasis was restricted to serve as passive Ca(2+) sinks that accumulate Ca(2+) along the organelle's negative membrane potential. This paradigm has changed radically. Nowadays, mitochondria are known to respond to environmental Ca(2+) and to contribute actively to the regulation of spatial and temporal patterns of intracellular Ca(2+) signaling. Accordingly, mitochondria contribute to many signal transduction pathways and are actively involved in the maintenance of capacitative Ca(2+) entry, the accomplishment of Ca(2+) refilling of the endoplasmic reticulum and Ca(2+)-dependent protein folding. Mitochondrial Ca(2+) homeostasis is complex and regulated by numerous, so far, genetically unidentified Ca(2+) channels, pumps and exchangers that concertedly accomplish the organelle's Ca(2+) demand. Notably, mitochondrial Ca(2+) homeostasis and functions are crucially influenced by the organelle's structural organization and motility that, in turn, is controlled by matrix/cytosolic Ca(2+). This review intends to provide a condensed overview on the molecular mechanisms of mitochondrial Ca(2+) homeostasis (uptake, buffering and storage, extrusion), its modulation by other ions, kinases and small molecules, and its contribution to cellular processes as fundamental basis for the organelle's contribution to signaling pathways. Hence, emphasis is given to the structure-to-function and mobility-to-function relationship of the mitochondria and, thereby, bridging our most recent knowledge on mitochondria with the best-established mitochondrial function: metabolism and ATP production.
Figures
References
-
- Alirol E, Martinou JC. Mitochondria and cancer: is there a morphological connection? Oncogene. 2006;25:4706–4716. - PubMed
-
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–214. - PubMed
-
- Armstrong JS. The role of the mitochondrial permeability transition in cell death. Mitochondrion. 2006;6:225–234. - PubMed
-
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

