Adenovirus type 5 E1A-induced apoptosis in COX-2-overexpressing breast cancer cells
- PMID: 17612393
- PMCID: PMC2206712
- DOI: 10.1186/bcr1739
Adenovirus type 5 E1A-induced apoptosis in COX-2-overexpressing breast cancer cells
Abstract
Introduction: Suppression of Bcl-2 expression can overcome cellular resistance to apoptosis induced by the adenovirus type 5 gene E1A in models of ovarian and breast cancer. Celecoxib, a cyclooxygenase-2 (COX-2) inhibitor, is known to downregulate Bcl-2 expression. We hypothesized that celecoxib would enhance E1A-induced apoptosis by suppressing Bcl-2 through suppressing COX-2 expression. If successful, this strategy could represent a means of overcoming resistance to E1A gene therapy.
Methods: We first established the cytotoxicity of celecoxib in two COX-2-overexpressing E1A-transfected breast cancer cell lines (MDA-MB-231 and MDA-MB-435) and in two low-COX-2-expressing E1A-transfected cell lines (MCF-7 (breast cancer) and SKOV3.ip1 (ovarian cancer)). We next tested whether higher sensitivity to celecoxib among these cell lines resulted from increased apoptosis by flow cytometry and western blotting. We further investigated whether suppression of Bcl-2 by celecoxib was involved in the apoptosis resulting from celecoxib treatment, and we explored whether the celecoxib-induced apoptosis in these cells depends on a COX-2 downstream pathway.
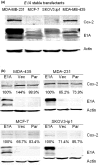
Results: The two COX-2-overexpressing cell lines MDA-MB-231-E1A and MDA-MB-435-E1A were more sensitive to celecoxib than the corresponding control cells, but the two low-COX-2-expressing cell lines MCF-7-E1A and SKOV3.ip1-E1A were no more sensitive than control cells to celecoxib. Therefore, we used the MDA-MB-231-E1A and MDA-MB-435-E1A cells for all further experiments. In both cell lines, sub-G1 fraction was increased, or cleavage of PARP and caspase-9 were increased after 5 days of exposure to 40 microM celecoxib. However, Bcl-2 was suppressed only in the MDA-MB-435-E1A cells and not in the MDA-MB-231-E1A cells. Restoring Bcl-2 expression in the MDA-MB-435-E1A stable transfectants did not affect their sensitivity to celecoxib. However, adding prostaglandin E2 (PGE2) or PGF2alpha blunted the sensitivity to celecoxib of both E1A stable transfectants.
Conclusion: We speculate that one mechanism by which celecoxib enhances E1A-induced apoptosis in cells that express high levels of COX-2 is through blocking PGE2 or PGF2alpha.
Figures



Similar articles
-
Celecoxib and Bcl-2: emerging possibilities for anticancer drug design.Future Med Chem. 2012 Mar;4(3):361-83. doi: 10.4155/fmc.11.177. Future Med Chem. 2012. PMID: 22393942 Free PMC article. Review.
-
Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells.Breast Cancer Res. 2005;7(4):R422-35. doi: 10.1186/bcr1019. Epub 2005 Apr 4. Breast Cancer Res. 2005. PMID: 15987447 Free PMC article.
-
Potent cell growth inhibitory effects in hepatitis B virus X protein positive hepatocellular carcinoma cells by the selective cyclooxygenase-2 inhibitor celecoxib.Mol Carcinog. 2009 Jan;48(1):56-65. doi: 10.1002/mc.20455. Mol Carcinog. 2009. PMID: 18506760
-
Cyclooxygenase-2 inhibitor celecoxib augments chemotherapeutic drug-induced apoptosis by enhancing activation of caspase-3 and -9 in prostate cancer cells.Int J Cancer. 2005 Jun 20;115(3):484-92. doi: 10.1002/ijc.20878. Int J Cancer. 2005. PMID: 15688368
-
Compositions for treatment of cancer and inflammation.Recent Pat Anticancer Drug Discov. 2008 Jan;3(1):55-62. doi: 10.2174/157489208783478720. Recent Pat Anticancer Drug Discov. 2008. PMID: 18289124 Review.
Cited by
-
BH3-based fusion artificial peptide induces apoptosis and targets human colon cancer.Mol Ther. 2009 Sep;17(9):1509-16. doi: 10.1038/mt.2009.43. Epub 2009 Apr 7. Mol Ther. 2009. PMID: 19352325 Free PMC article.
-
Cyclooxygenase-2 and the inflammogenesis of breast cancer.World J Clin Oncol. 2014 Oct 10;5(4):677-92. doi: 10.5306/wjco.v5.i4.677. World J Clin Oncol. 2014. PMID: 25302170 Free PMC article. Review.
-
Celecoxib and Bcl-2: emerging possibilities for anticancer drug design.Future Med Chem. 2012 Mar;4(3):361-83. doi: 10.4155/fmc.11.177. Future Med Chem. 2012. PMID: 22393942 Free PMC article. Review.
References
-
- Hortobagyi GN, Ueno NT, Xia W, Zhang S, Wolf JK, Putnam JB, Weiden PL, Willey JS, Carey M, Branham DL, et al. Cationic liposome-mediated E1A gene transfer to human breast and ovarian cancer cells and its biologic effects: a phase I clinical trial. J Clin Oncol. 2001;19:3422–3433. - PubMed
-
- Villaret D, Glisson B, Kenady D, Hanna E, Carey M, Gleich L, Yoo GH, Futran N, Hung MC, Anklesaria P, et al. A multicenter phase II study of tgDCC-E1A for the intratumoral treatment of patients with recurrent head and neck squamous cell carcinoma. Head Neck. 2002;24:661–669. doi: 10.1002/hed.10107. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

