Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes
- PMID: 17612411
- PMCID: PMC1940245
- DOI: 10.1186/1471-2148-7-109
Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes
Abstract
Background: NADPH-oxidases (Nox) and the related Dual oxidases (Duox) play varied biological and pathological roles via regulated generation of reactive oxygen species (ROS). Members of the Nox/Duox family have been identified in a wide variety of organisms, including mammals, nematodes, fruit fly, green plants, fungi, and slime molds; however, little is known about the molecular evolutionary history of these enzymes.
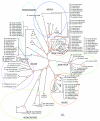
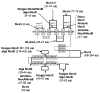
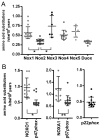
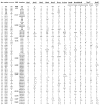
Results: We assembled and analyzed the deduced amino acid sequences of 101 Nox/Duox orthologs from 25 species, including vertebrates, urochordates, echinoderms, insects, nematodes, fungi, slime mold amoeba, alga and plants. In contrast to ROS defense enzymes, such as superoxide dismutase and catalase that are present in prokaryotes, ROS-generating Nox/Duox orthologs only appeared later in evolution. Molecular taxonomy revealed seven distinct subfamilies of Noxes and Duoxes. The calcium-regulated orthologs representing 4 subfamilies diverged early and are the most widely distributed in biology. Subunit-regulated Noxes represent a second major subdivision, and appeared first in fungi and amoeba. Nox5 was lost in rodents, and Nox3, which functions in the inner ear in gravity perception, emerged the most recently, corresponding to full-time adaptation of vertebrates to land. The sea urchin Strongylocentrotus purpuratus possesses the earliest Nox2 co-ortholog of vertebrate Nox1, 2, and 3, while Nox4 first appeared somewhat later in urochordates. Comparison of evolutionary substitution rates demonstrates that Nox2, the regulatory subunits p47phox and p67phox, and Duox are more stringently conserved in vertebrates than other Noxes and Nox regulatory subunits. Amino acid sequence comparisons identified key catalytic or regulatory regions, as 68 residues were highly conserved among all Nox/Duox orthologs, and 14 of these were identical with those mutated in Nox2 in variants of X-linked chronic granulomatous disease. In addition to canonical motifs, the B-loop, TM6-FAD, VXGPFG-motif, and extreme C-terminal regions were identified as important for Nox activity, as verified by mutational analysis. The presence of these non-canonical, but highly conserved regions suggests that all Nox/Duox may possess a common biological function remained in a long history of Nox/Duox evolution.
Conclusion: This report provides the first comprehensive analysis of the evolution and conserved functions of Nox and Duox family members, including identification of conserved amino acid residues. These results provide a guide for future structure-function studies and for understanding the evolution of biological functions of these enzymes.
Figures



Similar articles
-
Molecular evolution of Phox-related regulatory subunits for NADPH oxidase enzymes.BMC Evol Biol. 2007 Sep 27;7:178. doi: 10.1186/1471-2148-7-178. BMC Evol Biol. 2007. PMID: 17900370 Free PMC article.
-
NOX family NADPH oxidases: not just in mammals.Biochimie. 2007 Sep;89(9):1107-12. doi: 10.1016/j.biochi.2007.01.012. Epub 2007 Feb 20. Biochimie. 2007. PMID: 17400358 Review.
-
Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species.FEBS J. 2008 Jul;275(13):3249-77. doi: 10.1111/j.1742-4658.2008.06488.x. Epub 2008 May 30. FEBS J. 2008. PMID: 18513324 Review.
-
Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation.J Biol Chem. 2005 Sep 9;280(36):31859-69. doi: 10.1074/jbc.M501882200. Epub 2005 Jun 30. J Biol Chem. 2005. PMID: 15994299
-
[The Nox/Duox family of ROS-generating NADPH oxidases].Med Sci (Paris). 2006 Nov;22(11):953-9. doi: 10.1051/medsci/20062211953. Med Sci (Paris). 2006. PMID: 17101097 Review. French.
Cited by
-
Modeling Virus-Induced Inflammation in Zebrafish: A Balance Between Infection Control and Excessive Inflammation.Front Immunol. 2021 May 7;12:636623. doi: 10.3389/fimmu.2021.636623. eCollection 2021. Front Immunol. 2021. PMID: 34025644 Free PMC article. Review.
-
From Flies to Men: ROS and the NADPH Oxidase in Phagocytes.Front Cell Dev Biol. 2021 Mar 26;9:628991. doi: 10.3389/fcell.2021.628991. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33842458 Free PMC article. Review.
-
Extracellular superoxide dismutase inhibits hepatocyte growth factor-mediated breast cancer-fibroblast interactions.Oncotarget. 2017 Nov 10;8(64):107390-107408. doi: 10.18632/oncotarget.22379. eCollection 2017 Dec 8. Oncotarget. 2017. PMID: 29296173 Free PMC article.
-
Nox NADPH oxidases and the endoplasmic reticulum.Antioxid Redox Signal. 2014 Jun 10;20(17):2755-75. doi: 10.1089/ars.2013.5605. Epub 2014 Feb 26. Antioxid Redox Signal. 2014. PMID: 24386930 Free PMC article. Review.
-
Reactive Oxygen-Related Diseases: Therapeutic Targets and Emerging Clinical Indications.Antioxid Redox Signal. 2015 Nov 10;23(14):1171-85. doi: 10.1089/ars.2015.6433. Antioxid Redox Signal. 2015. PMID: 26583264 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

