Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection
- PMID: 17612487
- PMCID: PMC1986705
- DOI: 10.1016/j.molcel.2007.06.004
Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection
Abstract
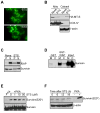
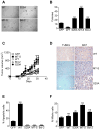
Cell death pathways are likely regulated in specialized subcellular microdomains, but how this occurs is not understood. Here, we show that cyclic AMP-dependent protein kinase A (PKA) phosphorylates the inhibitor of apoptosis (IAP) protein survivin on Ser20 in the cytosol, but not in mitochondria. This phosphorylation event disrupts the binding interface between survivin and its antiapoptotic cofactor, XIAP. Conversely, mitochondrial survivin or a non-PKA phosphorylatable survivin mutant binds XIAP avidly, enhances XIAP stability, synergistically inhibits apoptosis, and accelerates tumor growth, in vivo. Therefore, differential phosphorylation of survivin by PKA in subcellular microdomains regulates tumor cell apoptosis via its interaction with XIAP.
Figures
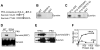




References
-
- Abrams JM. Competition and compensation: coupled to death in development and cancer. Cell. 2002;110:403–406. - PubMed
-
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. - PubMed
-
- Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. - PubMed
-
- Amieux PS, McKnight GS. The essential role of RI alpha in the maintenance of regulated PKA activity. Ann N Y Acad Sci. 2002;968:75–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

