A non-Q/N-rich prion domain of a foreign prion, [Het-s], can propagate as a prion in yeast
- PMID: 17612491
- PMCID: PMC1995001
- DOI: 10.1016/j.molcel.2007.05.027
A non-Q/N-rich prion domain of a foreign prion, [Het-s], can propagate as a prion in yeast
Abstract
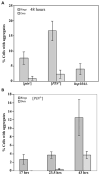
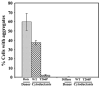
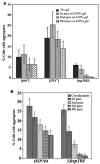
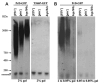
Prions are self-propagating, infectious aggregates of misfolded proteins. The mammalian prion, PrP(Sc), causes fatal neurodegenerative disorders. Fungi also have prions. While yeast prions depend upon glutamine/asparagine (Q/N)-rich regions, the Podospora anserina HET-s and PrP prion proteins lack such sequences. Nonetheless, we show that the HET-s prion domain fused to GFP propagates as a prion in yeast. Analogously to native yeast prions, transient overexpression of the HET-s fusion induces ring-like aggregates that propagate in daughter cells as cytoplasmically inherited, detergent-resistant dot aggregates. Efficient dot propagation, but not ring formation, is dependent upon the Hsp104 chaperone. The yeast prion [PIN(+)] enhances HET-s ring formation, suggesting that prions with and without Q/N-rich regions interact. Finally, HET-s aggregates propagated in yeast are infectious when introduced into Podospora. Taken together, these results demonstrate prion propagation in a truly foreign host. Since yeast can host non-Q/N-rich prions, such native yeast prions may exist.
Figures


Similar articles
-
Analyzing the birth and propagation of two distinct prions, [PSI+] and [Het-s](y), in yeast.Mol Biol Cell. 2010 May 1;21(9):1449-61. doi: 10.1091/mbc.e09-11-0927. Epub 2010 Mar 10. Mol Biol Cell. 2010. PMID: 20219972 Free PMC article.
-
Role of Hsp104 in the propagation and inheritance of the [Het-s] prion.Mol Biol Cell. 2007 Dec;18(12):4803-12. doi: 10.1091/mbc.e07-07-0657. Epub 2007 Sep 19. Mol Biol Cell. 2007. PMID: 17881723 Free PMC article.
-
The yeast global transcriptional co-repressor protein Cyc8 can propagate as a prion.Nat Cell Biol. 2009 Mar;11(3):344-9. doi: 10.1038/ncb1843. Epub 2009 Feb 15. Nat Cell Biol. 2009. PMID: 19219034 Free PMC article.
-
Infectious fold and amyloid propagation in Podospora anserina.Prion. 2007 Jan-Mar;1(1):44-7. doi: 10.4161/pri.1.1.4083. Epub 2007 Jan 28. Prion. 2007. PMID: 19164904 Free PMC article. Review.
-
Yeast prions: evolution of the prion concept.Prion. 2007 Apr-Jun;1(2):94-100. doi: 10.4161/pri.1.2.4664. Epub 2007 Apr 28. Prion. 2007. PMID: 19164928 Free PMC article. Review.
Cited by
-
The toxicity of an "artificial" amyloid is related to how it interacts with membranes.Prion. 2010 Oct-Dec;4(4):283-91. doi: 10.4161/pri.4.4.13126. Epub 2010 Oct 23. Prion. 2010. PMID: 21057225 Free PMC article.
-
Soluble oligomers are sufficient for transmission of a yeast prion but do not confer phenotype.J Cell Biol. 2013 Oct 28;203(2):197-204. doi: 10.1083/jcb.201307040. Epub 2013 Oct 21. J Cell Biol. 2013. PMID: 24145167 Free PMC article.
-
New insights into prion biology from the novel [SWI+] system.Prion. 2008 Oct-Dec;2(4):141-4. doi: 10.4161/pri.2.4.8069. Prion. 2008. PMID: 19256027 Free PMC article. Review.
-
The HET-S/s Prion Motif in the Control of Programmed Cell Death.Cold Spring Harb Perspect Biol. 2016 Sep 1;8(9):a023515. doi: 10.1101/cshperspect.a023515. Cold Spring Harb Perspect Biol. 2016. PMID: 27352624 Free PMC article. Review.
-
Functional Amyloids.Cold Spring Harb Perspect Biol. 2019 Dec 2;11(12):a033860. doi: 10.1101/cshperspect.a033860. Cold Spring Harb Perspect Biol. 2019. PMID: 31088827 Free PMC article. Review.
References
-
- Bagriantsev S, Liebman SW. Specificity of prion assembly in vivo. [PSI+] and [PIN+] form separate structures in yeast. J Biol Chem. 2004;279:51042–51048. - PubMed
-
- Baron T. Mouse models of prion disease transmission. Trends Mol Med. 2002;8:495–500. - PubMed
-
- Baxa U, Cassese T, Kajava AV, Steven AC. Structure, function, and amyloidogenesis of fungal prions: filament polymorphism and prion variants. Adv Protein Chem. 2006;73:125–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

