Initiation of RNA decay in Escherichia coli by 5' pyrophosphate removal
- PMID: 17612492
- PMCID: PMC2196405
- DOI: 10.1016/j.molcel.2007.05.038
Initiation of RNA decay in Escherichia coli by 5' pyrophosphate removal
Abstract
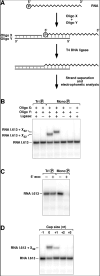
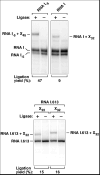
The common belief that endonucleolytic cleavage is the initial, rate-determining step of mRNA decay in Escherichia coli fails to explain the influence of 5' termini on the half-lives of primary transcripts. We have re-examined the initial events of RNA degradation in that organism by devising an assay to probe the 5' phosphorylation state of RNA and by employing a self-cleaving hammerhead ribozyme to investigate the degradative consequences of an unphosphorylated 5' end. These studies have identified a previously unrecognized prior step in decay that triggers subsequent internal cleavage by the endonuclease RNase E and thereby governs RNA longevity: the rate-determining conversion of a triphosphorylated to a monophosphorylated 5' terminus. Our findings redefine the role of RNase E in RNA degradation and explain how unpaired 5'-terminal nucleotides can facilitate access to internal cleavage sites within primary transcripts. Moreover, these results reveal a striking parallel between the mechanisms of mRNA decay in prokaryotic and eukaryotic organisms.
Figures





Similar articles
-
The bacterial enzyme RppH triggers messenger RNA degradation by 5' pyrophosphate removal.Nature. 2008 Jan 17;451(7176):355-8. doi: 10.1038/nature06475. Nature. 2008. PMID: 18202662
-
Differential control of the rate of 5'-end-dependent mRNA degradation in Escherichia coli.J Bacteriol. 2012 Nov;194(22):6233-9. doi: 10.1128/JB.01223-12. Epub 2012 Sep 14. J Bacteriol. 2012. PMID: 22984254 Free PMC article.
-
Dual-acting riboswitch control of translation initiation and mRNA decay.Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):E3444-53. doi: 10.1073/pnas.1214024109. Epub 2012 Nov 19. Proc Natl Acad Sci U S A. 2012. PMID: 23169642 Free PMC article.
-
Surprises at the 3' end of prokaryotic RNA.Cell. 1995 Mar 24;80(6):829-32. doi: 10.1016/0092-8674(95)90284-8. Cell. 1995. PMID: 7535193 Review. No abstract available.
-
Maturation and degradation of RNA in bacteria.Curr Opin Microbiol. 2007 Jun;10(3):271-8. doi: 10.1016/j.mib.2007.05.008. Epub 2007 Jun 7. Curr Opin Microbiol. 2007. PMID: 17560162 Review.
Cited by
-
Structures of RNA complexes with the Escherichia coli RNA pyrophosphohydrolase RppH unveil the basis for specific 5'-end-dependent mRNA decay.J Biol Chem. 2015 Apr 10;290(15):9487-99. doi: 10.1074/jbc.M114.634824. Epub 2015 Feb 5. J Biol Chem. 2015. PMID: 25657011 Free PMC article.
-
Protein-coding gene promoters in Methanocaldococcus (Methanococcus) jannaschii.Nucleic Acids Res. 2009 Jun;37(11):3588-601. doi: 10.1093/nar/gkp213. Epub 2009 Apr 9. Nucleic Acids Res. 2009. PMID: 19359364 Free PMC article.
-
Post-transcriptional control by bacteriophage T4: mRNA decay and inhibition of translation initiation.Virol J. 2010 Dec 3;7:360. doi: 10.1186/1743-422X-7-360. Virol J. 2010. PMID: 21129205 Free PMC article. Review.
-
The crystal structure of the Escherichia coli RNase E apoprotein and a mechanism for RNA degradation.Structure. 2008 Aug 6;16(8):1238-44. doi: 10.1016/j.str.2008.04.017. Structure. 2008. PMID: 18682225 Free PMC article.
-
Involvement of rppH in thermoregulation in Pseudomonas syringae.J Bacteriol. 2014 Jun;196(12):2313-22. doi: 10.1128/JB.00057-14. Epub 2014 Apr 11. J Bacteriol. 2014. PMID: 24727227 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

