SIRT1 regulates the function of the Nijmegen breakage syndrome protein
- PMID: 17612497
- PMCID: PMC2679807
- DOI: 10.1016/j.molcel.2007.05.029
SIRT1 regulates the function of the Nijmegen breakage syndrome protein
Abstract
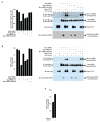
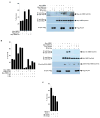
MRE11-RAD50-NBS1 (MRN) is a conserved nuclease complex that exhibits properties of a DNA damage sensor and is critical in regulating cellular responses to DNA double-strand breaks. NBS1, which is mutated in the human genetic disease Nijmegen breakage syndrome, serves as the regulatory subunit of MRN. Phosphorylation of NBS1 by the ATM kinase is necessary for both activation of the S phase checkpoint and for efficient DNA damage repair response. Here, we report that NBS1 is an acetylated protein and that the acetylation level is tightly regulated by the SIRT1 deacetylase. SIRT1 associates with the MRN complex and, importantly, maintains NBS1 in a hypoacetylated state, which is required for ionizing radiation-induced NBS1 Ser343 phosphorylation. Our results demonstrate the presence of crosstalk between two different posttranslational modifications in NBS1 and strongly suggest that deacetylation of NBS1 by SIRT1 plays a key role in the dynamic regulation of the DNA damage response and in the maintenance of genomic stability.
Figures




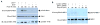
Similar articles
-
Distinct functions of Nijmegen breakage syndrome in ataxia telangiectasia mutated-dependent responses to DNA damage.Mol Cancer Res. 2003 Jul;1(9):674-81. Mol Cancer Res. 2003. PMID: 12861053
-
NBS1 is regulated by two kind of mechanisms: ATM-dependent complex formation with MRE11 and RAD50, and cell cycle-dependent degradation of protein.J Radiat Res. 2017 Jul 1;58(4):487-494. doi: 10.1093/jrr/rrx014. J Radiat Res. 2017. PMID: 28369484 Free PMC article.
-
AsSIRTing the DNA damage response.Trends Cell Biol. 2008 Feb;18(2):77-83. doi: 10.1016/j.tcb.2007.11.007. Epub 2008 Jan 22. Trends Cell Biol. 2008. PMID: 18215521 Free PMC article. Review.
-
Distinct functional domains of Nbs1 modulate the timing and magnitude of ATM activation after low doses of ionizing radiation.Oncogene. 2004 Apr 15;23(17):3122-7. doi: 10.1038/sj.onc.1207447. Oncogene. 2004. PMID: 15048089
-
A functional link between SIRT1 deacetylase and NBS1 in DNA damage response.Cell Cycle. 2007 Dec 1;6(23):2869-71. doi: 10.4161/cc.6.23.5026. Cell Cycle. 2007. PMID: 18156798 Review.
Cited by
-
MPP8 and SIRT1 crosstalk in E-cadherin gene silencing and epithelial-mesenchymal transition.EMBO Rep. 2015 Jun;16(6):689-99. doi: 10.15252/embr.201439792. Epub 2015 Apr 13. EMBO Rep. 2015. PMID: 25870236 Free PMC article.
-
Sirtuins, bioageing, and cancer.J Aging Res. 2011;2011:235754. doi: 10.4061/2011/235754. Epub 2011 Jun 30. J Aging Res. 2011. PMID: 21766030 Free PMC article.
-
SIRT1/PARP1 crosstalk: connecting DNA damage and metabolism.Genome Integr. 2013 Dec 20;4(1):6. doi: 10.1186/2041-9414-4-6. Genome Integr. 2013. PMID: 24360018 Free PMC article.
-
The dual role of sirtuins in cancer.Genes Cancer. 2011 Jun;2(6):648-62. doi: 10.1177/1947601911417862. Genes Cancer. 2011. PMID: 21941620 Free PMC article.
-
SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging.Cell. 2008 Nov 28;135(5):907-18. doi: 10.1016/j.cell.2008.10.025. Cell. 2008. PMID: 19041753 Free PMC article.
References
-
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. - PubMed
-
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. - PubMed
-
- Blander G, Olejnik J, Krzymanska-Olejnik E, McDonagh T, Haigis M, Yaffe MB, Guarente L. SIRT1 shows no substrate specificity in vitro. J Biol Chem. 2005;280:9780–9785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

