Switching tumour necrosis factor alpha antagonists in patients with ankylosing spondylitis and psoriatic arthritis: an observational study over a 5-year period
- PMID: 17613555
- PMCID: PMC1994295
- DOI: 10.1136/ard.2007.073569
Switching tumour necrosis factor alpha antagonists in patients with ankylosing spondylitis and psoriatic arthritis: an observational study over a 5-year period
Abstract
Objective: To evaluate the clinical response after switching from one tumour necrosis factor (TNF)alpha antagonist to another in patients with ankylosing spondylitis (AS) and psoriatic arthritis (PsA).
Methods: In this ongoing, longitudinal, observational study, data were prospectively collected on efficacy and safety since 2000 for patients starting biological treatments. The present analysis was restricted to patients with a diagnosis of spondyloarthropathy (SpA) who switched from one TNFalpha antagonist to another because of inadequate efficacy or adverse events.
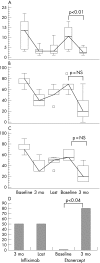
Results: In total, 589 anti-TNFalpha-naive patients were registered, of whom 165 had a diagnosis of SpA; 7 patients with AS and 15 with PsA received >1 TNFalpha antagonist. Two patients with PsA were treated with all the drugs. In all, 16 subjects switched from infliximab to etanercept, 7 from etanercept to adalimumab and 1 from etanercept to infliximab. Overall, a clinical response was seen in 75% of patients who changed from infliximab to etanercept, and in 57.1% who switched from etanercept to adalimumab.
Conclusions: The findings of this study on a selected population of patients with SpA indicate that the failure of an initial TNFalpha antagonist does not preclude the response to another one. Further trials are needed to confirm this preliminary observation.
Conflict of interest statement
Competing interests: None declared.
Similar articles
-
Costs of tumor necrosis factor blockers per treated patient using real-world drug data in a managed care population.J Manag Care Pharm. 2013 Oct;19(8):621-30. doi: 10.18553/jmcp.2013.19.8.621. J Manag Care Pharm. 2013. PMID: 24074008 Free PMC article.
-
Effectiveness and safety of adalimumab in patients with ankylosing spondylitis or psoriatic arthritis and history of anti-tumor necrosis factor therapy.Arthritis Res Ther. 2010;12(3):R117. doi: 10.1186/ar3054. Epub 2010 Jun 16. Arthritis Res Ther. 2010. PMID: 20553600 Free PMC article. Clinical Trial.
-
Observational study of switching anti-TNF agents in ankylosing spondylitis and psoriatic arthritis versus rheumatoid arthritis.Wien Med Wochenschr. 2010 May;160(9-10):220-4. doi: 10.1007/s10354-010-0795-0. Wien Med Wochenschr. 2010. PMID: 20632149
-
[Anti-TNF alpha in the treatment of psoriatic arthritis].Presse Med. 2006 Apr;35(4 Pt 2):647-55. doi: 10.1016/s0755-4982(06)74658-9. Presse Med. 2006. PMID: 16614610 Review. French.
-
TNF alpha inhibition as treatment modality for certain rheumatologic and gastrointestinal diseases.Endocr Metab Immune Disord Drug Targets. 2009 Sep;9(3):295-314. doi: 10.2174/187153009789044347. Epub 2009 Sep 1. Endocr Metab Immune Disord Drug Targets. 2009. PMID: 19594416 Review.
Cited by
-
Effect of Upadacitinib on Disease Activity, Pain, Fatigue, Function, Health-Related Quality of Life and Work Productivity for Biologic Refractory Ankylosing Spondylitis.Rheumatol Ther. 2023 Jun;10(3):679-691. doi: 10.1007/s40744-023-00536-2. Epub 2023 Feb 23. Rheumatol Ther. 2023. PMID: 36820984 Free PMC article.
-
Psoriatic arthritis: review of potential biomarkers predicting response to TNF inhibitors.Inflammopharmacology. 2023 Feb;31(1):77-87. doi: 10.1007/s10787-022-01092-x. Epub 2022 Dec 12. Inflammopharmacology. 2023. PMID: 36508130 Free PMC article. Review.
-
Efficacy and safety of upadacitinib, a selective JAK-1 inhibitor in treatment of ankylosing spondylitis: a meta-analysis.BMC Rheumatol. 2025 Feb 18;9(1):19. doi: 10.1186/s41927-025-00467-1. BMC Rheumatol. 2025. PMID: 39966910 Free PMC article.
-
Health care utilization and cost associated with switching biologics within the first year of biologic treatment initiation among patients with ankylosing spondylitis.J Manag Care Spec Pharm. 2021 Jan;27(1):27-36. doi: 10.18553/jmcp.2020.19433. Epub 2020 Oct 12. J Manag Care Spec Pharm. 2021. PMID: 33043820 Free PMC article.
-
Effectiveness and safety of ustekinumab in naïve or TNF-inhibitors failure psoriatic arthritis patients: a 24-month prospective multicentric study.Clin Rheumatol. 2018 Feb;37(2):397-405. doi: 10.1007/s10067-017-3953-6. Epub 2018 Jan 4. Clin Rheumatol. 2018. PMID: 29302829
References
-
- Kavanaugh A, Tutuncu Z, Catalan‐Sanchez T. Update on anti‐tumor necrosis factor therapy in the spondyloarthropathies including psoriatic arthritis. Curr Opin Rheumatol 200618347–353. - PubMed
-
- van Vollenhoven R F. Switching between biological agents. Clin Exp Rheumatol 200422(Suppl)S115–S121. - PubMed
-
- Wick M C, Ernestam S, Lindblad S, Bratt J, Klareskog L, van Vollenhoven R F. Adalimumab (Humira®) restores clinical response in patients with secondary loss of efficacy from infliximab (Remicade®) or etanercept (Enbrel®): results from the STURE registry at Karolinska University Hospital. Scand J Rheumatol 200534353–358. - PubMed
-
- Hyrich K L, Lunt M, Watson K D, Deborah P, Symmons M, Silman AJ for the British Society for Rheumatology Biologics Register Outcomes after switching from one anti‐tumor necrosis factor α agent to a second anti‐tumor necrosis factor α agent in patients with rheumatoid arthritis. Results from a large UK national cohort study. Arthritis Rheum 20075613–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous