Androgen receptors in a cichlid fish, Astatotilapia burtoni: structure, localization, and expression levels
- PMID: 17614300
- PMCID: PMC2743600
- DOI: 10.1002/cne.21435
Androgen receptors in a cichlid fish, Astatotilapia burtoni: structure, localization, and expression levels
Abstract
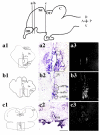
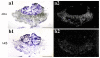
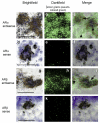
Androgens are an important output of the hypothalamic-pituitary-gonadal (HPG) axis that controls reproduction in all vertebrates. In male teleosts two androgens, testosterone and 11-ketotestosterone, control sexual differentiation and development in juveniles and reproductive behavior in adults. Androgenic signals provide feedback at many levels of the HPG axis, including the hypothalamic neurons that synthesize and release gonadotropin-releasing hormone 1 (GnRH1), but the precise cellular site of androgen action in the brain is not known. Here we describe two androgen receptor subtypes, ARalpha and ARbeta, in the cichlid Astatotilapia burtoni and show that these subtypes are differentially located throughout the adult brain in nuclei known to function in the control of reproduction. ARalpha was expressed in the ventral part of the ventral telencephalon, the preoptic area (POA) of the hypothalamus and the ventral hypothalamus, whereas ARbeta was more widely expressed in the dorsal and ventral telencephalon, the POA, and the ventral and dorsal hypothalamus. We provide the first evidence in any vertebrate that the GnRH1-releasing neurons, which serve as the central control point of the HPG axis, express both subtypes of AR. Using quantitative real-time PCR, we show that A. burtoni AR subtypes have different expression levels in adult tissue, with ARalpha showing significantly higher expression than ARbeta in the pituitary, and ARbeta expressed at a higher level than ARalpha in the anterior and middle brain. These data provide important insight into the role of androgens in regulating the vertebrate reproductive axis.
(c) 2007 Wiley-Liss, Inc.
Figures




Similar articles
-
Expression of novel androgen receptors in three GnRH neuron subtypes in the cichlid brain.J Neuroendocrinol. 2024 Nov;36(11):e13429. doi: 10.1111/jne.13429. Epub 2024 Jul 10. J Neuroendocrinol. 2024. PMID: 38986626
-
Social regulation of cortisol receptor gene expression.J Exp Biol. 2014 Sep 15;217(Pt 18):3221-8. doi: 10.1242/jeb.104430. Epub 2014 Jul 10. J Exp Biol. 2014. PMID: 25013108 Free PMC article.
-
Expression, structure, function, and evolution of gonadotropin-releasing hormone (GnRH) receptors GnRH-R1SHS and GnRH-R2PEY in the teleost, Astatotilapia burtoni.Endocrinology. 2007 Oct;148(10):5060-71. doi: 10.1210/en.2006-1400. Epub 2007 Jun 26. Endocrinology. 2007. PMID: 17595228
-
Androgen signaling in male fishes: Examples of anti-androgenic chemicals that cause reproductive disorders.Theriogenology. 2019 Nov;139:58-71. doi: 10.1016/j.theriogenology.2019.07.020. Epub 2019 Jul 19. Theriogenology. 2019. PMID: 31369937 Review.
-
Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis.Physiology (Bethesda). 2011 Dec;26(6):412-23. doi: 10.1152/physiol.00032.2011. Physiology (Bethesda). 2011. PMID: 22170959 Review.
Cited by
-
Female-specific target sites for both oestrogen and androgen in the teleost brain.Proc Biol Sci. 2012 Dec 22;279(1749):5014-23. doi: 10.1098/rspb.2012.2011. Epub 2012 Oct 17. Proc Biol Sci. 2012. PMID: 23075834 Free PMC article.
-
Modular genetic control of social status in a cichlid fish.Proc Natl Acad Sci U S A. 2020 Nov 10;117(45):28167-28174. doi: 10.1073/pnas.2008925117. Epub 2020 Oct 26. Proc Natl Acad Sci U S A. 2020. PMID: 33106426 Free PMC article.
-
Steroid Transport, Local Synthesis, and Signaling within the Brain: Roles in Neurogenesis, Neuroprotection, and Sexual Behaviors.Front Neurosci. 2018 Feb 20;12:84. doi: 10.3389/fnins.2018.00084. eCollection 2018. Front Neurosci. 2018. PMID: 29515356 Free PMC article. Review.
-
Neural and hormonal mechanisms of reproductive-related arousal in fishes.Horm Behav. 2011 May;59(5):616-29. doi: 10.1016/j.yhbeh.2010.10.006. Epub 2010 Oct 13. Horm Behav. 2011. PMID: 20950618 Free PMC article. Review.
-
Evolutionary Fate of the Androgen Receptor-Signaling Pathway in Ray-Finned Fishes with a Special Focus on Cichlids.G3 (Bethesda). 2015 Sep 1;5(11):2275-83. doi: 10.1534/g3.115.020685. G3 (Bethesda). 2015. PMID: 26333839 Free PMC article.
References
-
- Amano M, Hyodo S, Urano A, Okumoto N, Kitamura S, Ikuta K, Suzuki Y, Aida K. Activation of salmon gonadotropin-releasing hormone synthesis by 17 alpha-methyltestosterone administration in yearling masu salmon, Oncorhynchus masou. Gen Comp Endocrinol. 1994;95:374–380. - PubMed
-
- Ando H, Hasegawa M, Ando J, Urano A. Expression of salmon corticotropin-releasing hormone precursor gene in the preoptic nucleus in stressed rainbow trout. Gen Comp Endocrinol. 1999;113:87–95. - PubMed
-
- Antonopoulou E, Swanson P, Mayer I, Borg B. Feedback control of gonadotropins in Atlantic salmon, Salmo salar, male. Part II. Aromatase inhibitor and androgen effects. Gen Comp Endocrinol. 1999;114:142–150. - PubMed
-
- Bakker J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J Neuroendocrinol. 2003;15:615–621. - PubMed
-
- Belsham DD, Lovejoy DA. Gonadotropin-releasing hormone: gene evolution, expression, and regulation. Vitam Horm. 2005;71:59–94. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

