Studies of the role of ubiquitination in the interaction of ubiquilin with the loop and carboxyl terminal regions of presenilin-2
- PMID: 17614368
- PMCID: PMC2547082
- DOI: 10.1021/bi700604q
Studies of the role of ubiquitination in the interaction of ubiquilin with the loop and carboxyl terminal regions of presenilin-2
Abstract
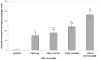
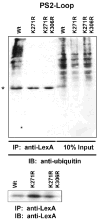
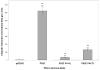
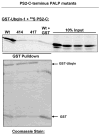
Ubiquilin was originally identified as a presenilin-interacting protein. We previously reported that ubiquilin interacts with both the loop and carboxyl terminus of presenilin proteins and that the ubiquitin-associated (UBA) domain of ubiquilin, which binds poly ubiquitin chains, is important for mediating this interaction. In the present study, we examined whether ubiquitination of presenilin-2 (PS2) is required for interaction with ubiquilin-1 by mutating lysine residues that may be targets for ubiquitination in the presenilin loop and carboxyl terminus regions. Mutation of two lysine residues in the PS2-loop region suggested that ubiquitination is not required for interaction with ubiquilin-1 and may, in fact, even negatively regulate the interaction. Similarly, we found that ubiquitination of the PS2 carboxyl terminus (PS2-C-terminus) is not required for interaction with ubiquilin-1, although our results suggest that it could play some role. Instead, we found that the mutation of either one of the two lysine residues in the carboxyl terminus of PS2 or the proline residues in the highly conserved PALP motif in this region results in destabilization of the mutant PS2 polypeptides because of increased degradation by the proteasome. Furthermore, by GST-pull-down assays we found that the mutant polypeptides were unable to bind ubiquilin, suggesting that loss of ubiquilin interaction leads to destabilization of presenilin polypeptides. Paradoxically, however, knockdown of ubiquilin expression by RNA interference did not alter the rate of turnover of PS2 proteins in cells. Instead, we found that PS2 synthesis was reduced, and PS2 fragment production was increased, suggesting that ubiquilin expression modulates biogenesis and endoproteolysis of presenilin proteins.
Figures

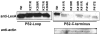




Similar articles
-
Ubiquilin regulates presenilin endoproteolysis and modulates gamma-secretase components, Pen-2 and nicastrin.Biochem J. 2005 Nov 1;391(Pt 3):513-25. doi: 10.1042/BJ20050491. Biochem J. 2005. PMID: 15975090 Free PMC article.
-
Overexpression of ubiquilin decreases ubiquitination and degradation of presenilin proteins.J Alzheimers Dis. 2004 Feb;6(1):79-92. doi: 10.3233/jad-2004-6109. J Alzheimers Dis. 2004. PMID: 15004330
-
Identification of ubiquilin, a novel presenilin interactor that increases presenilin protein accumulation.J Cell Biol. 2000 Nov 13;151(4):847-62. doi: 10.1083/jcb.151.4.847. J Cell Biol. 2000. PMID: 11076969 Free PMC article.
-
Emerging role of Alzheimer's disease-associated ubiquilin-1 in protein aggregation.Biochem Soc Trans. 2010 Feb;38(Pt 1):150-5. doi: 10.1042/BST0380150. Biochem Soc Trans. 2010. PMID: 20074050 Review.
-
Ubiquilin 2: a component of the ubiquitin-proteasome system with an emerging role in neurodegeneration.Int J Biochem Cell Biol. 2014 May;50:123-6. doi: 10.1016/j.biocel.2014.02.018. Epub 2014 Feb 28. Int J Biochem Cell Biol. 2014. PMID: 24589709 Review.
Cited by
-
Signature changes in ubiquilin expression in the R6/2 mouse model of Huntington's disease.Brain Res. 2015 Feb 9;1597:37-46. doi: 10.1016/j.brainres.2014.12.008. Epub 2014 Dec 12. Brain Res. 2015. PMID: 25511991 Free PMC article.
-
Inhibition of the Neuronal Calcium Sensor DREAM Modulates Presenilin-2 Endoproteolysis.Front Mol Neurosci. 2018 Dec 3;11:449. doi: 10.3389/fnmol.2018.00449. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30559648 Free PMC article.
-
Ubiquitin-like and ubiquitin-associated domain proteins: significance in proteasomal degradation.Cell Mol Life Sci. 2009 Sep;66(17):2819-33. doi: 10.1007/s00018-009-0048-9. Epub 2009 May 26. Cell Mol Life Sci. 2009. PMID: 19468686 Free PMC article. Review.
-
Alzheimer's disease-associated ubiquilin-1 regulates presenilin-1 accumulation and aggresome formation.Traffic. 2011 Mar;12(3):330-48. doi: 10.1111/j.1600-0854.2010.01149.x. Epub 2011 Jan 7. Traffic. 2011. PMID: 21143716 Free PMC article.
-
Defective Proteasome Delivery of Polyubiquitinated Proteins by Ubiquilin-2 Proteins Containing ALS Mutations.PLoS One. 2015 Jun 15;10(6):e0130162. doi: 10.1371/journal.pone.0130162. eCollection 2015. PLoS One. 2015. PMID: 26075709 Free PMC article.
References
-
- Conklin D, Holderman S, Whitmore TE, Maurer M, Feldhaus AL. Molecular cloning, chromosome mapping and characterization of UBQLN3 a testis-specific gene that contains an ubiquitin-like domain. Gene. 2000;249:91–98. - PubMed
-
- Wu S, Mikhailov A, Kallo-Hosein H, Hara K, Yonezawa K, Avruch J. Characterization of ubiquilin 1, an mTOR-interacting protein. Biochim Biophys Acta. 2002;1542:41–56. - PubMed
-
- Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. - PubMed
-
- Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

