The roles of adenosine and related substances in exercise hyperaemia
- PMID: 17615100
- PMCID: PMC2277189
- DOI: 10.1113/jphysiol.2007.136416
The roles of adenosine and related substances in exercise hyperaemia
Abstract
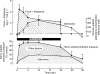
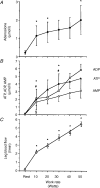
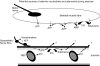
The role of adenosine in exercise hyperaemia has been controversial. Accumulating evidence now demonstrates that adenosine is released into the venous efflux of exercising muscle and that adenosine is responsible for 20-40% of the maintained phase of the muscle vasodilatation that accompanies submaximal and maximal contractions. This adenosine is mainly generated from AMP that is released from the skeletal muscle fibres and dephosphorylated by ecto 5'nucleotidase bound to the sarcolemma. During exercise, the concentration of ecto 5'nucleotidase may be increased by translocation from the cytosol, while release of AMP and affinity of ecto 5'nucleotidase for AMP are increased by acidosis. The adenosine so formed, acts on extraluminal A(2A) receptors on the vascular smooth muscle. In addition, ATP is released from red blood cells into the plasma during exercise, in association with the unloading of O(2) from haemoglobin, while ATP and adenosine may be released from endothelium as a consequence of local hypoxia. It is unlikely that this intraluminal ATP, or adenosine, contributes significantly to exercise hyperaemia, for muscle vasodilatation induced by intraluminal ATP or adenosine is strongly nitric oxide dependent, while vasodilatation induced by adenosine in hypoxia is mediated by A(1) receptors. Neither is a recognized feature of exercise hyperaemia.
Figures
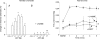
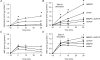
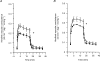
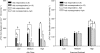
Similar articles
-
Nitric oxide (NO) does not contribute to the generation or action of adenosine during exercise hyperaemia in rat hindlimb.J Physiol. 2009 Apr 1;587(Pt 7):1579-91. doi: 10.1113/jphysiol.2008.163691. Epub 2009 Feb 9. J Physiol. 2009. PMID: 19204054 Free PMC article.
-
Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia.J Physiol. 1996 Sep 1;495 ( Pt 2)(Pt 2):553-60. doi: 10.1113/jphysiol.1996.sp021615. J Physiol. 1996. PMID: 8887765 Free PMC article.
-
Contribution of non-endothelium-dependent substances to exercise hyperaemia: are they O(2) dependent?J Physiol. 2012 Dec 15;590(24):6307-20. doi: 10.1113/jphysiol.2012.240721. Epub 2012 Oct 8. J Physiol. 2012. PMID: 23045341 Free PMC article. Review.
-
Contribution of intravascular versus interstitial purines and nitric oxide in the regulation of exercise hyperaemia in humans.J Physiol. 2012 Oct 15;590(20):5015-23. doi: 10.1113/jphysiol.2012.234963. Epub 2012 Jun 25. J Physiol. 2012. PMID: 22733661 Free PMC article. Review.
-
Adenosine and nitric oxide in exercise-induced human skeletal muscle vasodilatation.Acta Physiol Scand. 2000 Apr;168(4):575-91. doi: 10.1046/j.1365-201x.2000.00705.x. Acta Physiol Scand. 2000. PMID: 10759594 Review.
Cited by
-
Effect of acute sympathetic activation on leg vasodilation before and after endurance exercise.J Smooth Muscle Res. 2021;57(0):53-67. doi: 10.1540/jsmr.57.. J Smooth Muscle Res. 2021. PMID: 34789634 Free PMC article.
-
Potassium inhibits nitric oxide and adenosine arteriolar vasodilatation via K(IR) and Na(+)/K(+) ATPase: implications for redundancy in active hyperaemia.J Physiol. 2015 Dec 1;593(23):5111-26. doi: 10.1113/JP270613. Epub 2015 Nov 15. J Physiol. 2015. PMID: 26426256 Free PMC article.
-
Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs.Physiol Rev. 2015 Apr;95(2):549-601. doi: 10.1152/physrev.00035.2013. Physiol Rev. 2015. PMID: 25834232 Free PMC article. Review.
-
The Acute Effect of Hyperoxia on Onset of Blood Lactate Accumulation (OBLA) and Performance in Female Runners during the Maximal Treadmill Test.Int J Environ Res Public Health. 2021 Apr 25;18(9):4546. doi: 10.3390/ijerph18094546. Int J Environ Res Public Health. 2021. PMID: 33922940 Free PMC article.
-
Effects of modest hyperoxia and oral vitamin C on exercise hyperaemia and reactive hyperaemia in healthy young men.Eur J Appl Physiol. 2015 Sep;115(9):1995-2006. doi: 10.1007/s00421-015-3182-0. Epub 2015 May 12. Eur J Appl Physiol. 2015. PMID: 25963380 Clinical Trial.
References
-
- Belloni FL, Phair RD, Sparks HV. The role of adenosine in prolonged vasodilatation following flow restriction exercise of canine skeletal muscle. Circ Res. 1979;44:759–766. - PubMed
-
- Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–322. - PubMed
-
- Berne RM, Rubio R, Dobson JG, Curnish RR. Adenosine and adenine nucleotides as possible mediators of cardiac and skeletal muscle blood flow regulation. Circ Res. 1971;28–29(Suppl. 1):115–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

