Abl silencing inhibits CAS-mediated process and constriction in resistance arteries
- PMID: 17615370
- PMCID: PMC2084484
- DOI: 10.1161/CIRCRESAHA.107.156463
Abl silencing inhibits CAS-mediated process and constriction in resistance arteries
Abstract
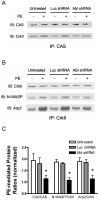
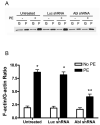
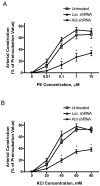
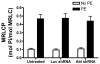
The tyrosine phosphorylated protein Crk-associated substrate (CAS) has previously been shown to participate in the cellular processes regulating dynamic changes in the actin architecture and arterial constriction. In the present study, treatment of rat mesenteric arteries with phenylephrine (PE) led to the increase in CAS tyrosine phosphorylation and the association of CAS with the adapter protein CrkII. CAS phosphorylation was catalyzed by Abl in an in vitro study. To determine the role of Abl tyrosine kinase in arterial vessels, plasmids encoding Abl short hairpin RNA (shRNA) were transduced into mesenteric arteries by chemical loading plus liposomes. Abl silencing diminished increases in CAS phosphorylation on PE stimulation. Previous studies have shown that assembly of the multiprotein compound containing CrkII, neuronal Wiskott-Aldrich Syndrome Protein (N-WASP) and the Arp2/3 (Actin Related Protein) complex triggers actin polymerization in smooth muscle as well as in nonmuscle cells. In this study, Abl silencing attenuated the assembly of the multiprotein compound in resistance arteries on contractile stimulation. Furthermore, the increase in F/G-actin ratios (an index of actin assembly) and constriction on contractile stimulation were reduced in Abl-deficient arterial segments compared with control arteries. However, myosin regulatory light chain phosphorylation (MRLCP) elicited by contractile activation was not inhibited in Abl-deficient arteries. These results suggest that Abl may play a pivotal role in mediating CAS phosphorylation, the assembly of the multiprotein complex, actin assembly, and constriction in resistance arteries. Abl does not participate in the regulation of myosin activation in arterial vessels during contractile stimulation.
Figures







References
-
- Tang DD, Zhang W, Gunst SJ. The Adapter Protein CrkII Regulates Neuronal Wiskott-Aldrich Syndrome Protein, Actin Polymerization, and Tension Development during Contractile Stimulation of Smooth Muscle. J Biol Chem. 2005 June 17;280(24):23380–9. - PubMed
-
- Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem. 2004 December 10;279(50):51722–8. - PubMed
-
- Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol. 2005 May;288(5):C1145–C1160. - PubMed
-
- Barany M, Barron JT, Gu L, Barany K. Exchange of the actin-bound nucleotide in intact arterial smooth muscle. J Biol Chem. 2001 December 21;276(51):48398–403. - PubMed
-
- Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002 January;16(1):72–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

