Human embryonic and neuronal stem cell markers in retinoblastoma
- PMID: 17615543
- PMCID: PMC2768758
Human embryonic and neuronal stem cell markers in retinoblastoma
Abstract
Purpose: Retinoblastoma (RB) is the most common intraocular tumor of early childhood. The early onset of RB, coupled with our previous findings of cancer stem cell characteristics in RB, led us to hypothesize that subpopulations of RB tumors harbor markers and behaviors characteristic of embryonic and neuronal origin.
Methods: Our RB sources included: human pathological tissues, and the human RB cell lines Y79 and WERI-RB27. Microarray screening, single and dual-label immunocytochemistry and RT-PCR were performed to detect embryonic and neuronal stem cell markers, such as Oct3/4, Nanog, CD133, and Musashi-1. To test for functional evidence of stem cell behavior, we examined RB cells for their ability to form neurospheres and retain BrdU label as indicators of self-renewal and slow cell cycling, respectively.
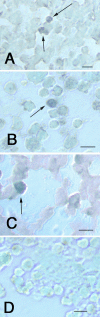
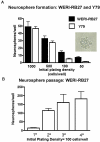
Results: Microarray comparisons of human RB tumors with normal retinal tissue detected upregulation of a number of genes involved in embryonic development that were also present in Y79 cells, including Oct3/4, Nanog, Musashi-1 and Musashi-2, prominin-1 (CD133), Jagged-2, Reelin, Thy-1, nestin, Meis-1,NCAM, Patched, and Notch4. Expression of Musashi-1, Oct3/4 and Nanog was confirmed by immunostaining and RT-PCR analyses of RB tumors and RB cell lines. CD133 expression was confirmed by PCR analysis. Y79 and WERI-RB27 contained populations of Hoechst-dim/ABCG2-positive cells that co-localized with embryonic stem cell markers Oct3/4-ABCG2 and Nanog-ABCG2. Subpopulations of Y79 and WERI-RB27 cells were label-retaining (as seen by BrdU incorporation) and were able to generate neurospheres, both hallmarks of a stem cell phenotype.
Conclusions: Small subpopulation(s) of RB cells express human embryonic and neuronal stem cell markers. There are also subpopulations that demonstrate functional behavior (label retention and self-renewal) consistent with cancer stem cells. These findings support the hypothesis that RB is a heterogeneous tumor comprised of subpopulation(s) with stem cell-like properties.
Figures




References
-
- Gallie BL, Dunn JM, Chan HS, Hamel PA, Phillips RA. The genetics of retinoblastoma. Relevance to the patient. Pediatr Clin North Am. 1991;38:299–315. - PubMed
-
- Abramson DH, Melson MR, Dunkel IJ, Frank CM. Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Ophthalmology. 2001;108:1868–76. - PubMed
-
- Wong FL, Boice JD, Jr, Abramson DH, Tarone RE, Kleinerman RA, Stovall M, Goldman MB, Seddon JM, Tarbell N, Fraumeni JF, Jr, Li FP. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA. 1997;278:1262–7. - PubMed
-
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
