Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity
- PMID: 17615545
- PMCID: PMC2770201
Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity
Abstract
Purpose: To study the mechanisms of action of the antioxidants, n-acetylcysteine (NAC), and the nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase oxidase inhibitor, apocynin, on intravitreous neovascularization (IVNV), and retinal avascularity in a rat model of retinopathy of prematurity (ROP).
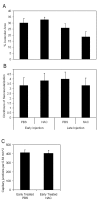
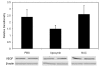
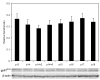
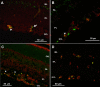
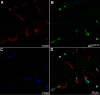
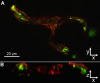
Methods: Newborn rats exposed to oxygen-induced retinopathy underwent intraperitoneal (IP) injections of NAC (150 mg/kg) at post-natal day (p)2, p6, p10 (early NAC-treated), or p12 through p17 (late NAC-treated), apocynin (10 mg/kg) from p12 through p17, or phosphate buffered saline (PBS; controls). Lipid hydroperoxide (LHP) was measured in early NAC-treated oxygen-induced retinopathy (OIR) at p7, p14 and p18. Pups were placed in room air at p14. At p18, retinal flat mounts were scored for IVNV and avascular/total retinal area, or retinas were assayed for cleaved caspase-3 and vascular endothelial growth factor (VEGF) protein. In non-injected OIR pups, retinas were assayed for gp91(phox). Cryosections were stained with isolectin B4, cleaved caspase-3, CD68, CD31, gp91(phox), neuron-glial antigen 2 (NG-2), or anti-glial fibrillary acidic protein (GFAP) and visualized with confocal microscopy.
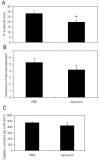
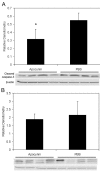
Results: LHP increased over time in retinas from OIR exposed pups in association with IVNV. Early NAC-treated retinas had significantly reduced LHP compared to PBS-control at p18 (p<0.012). However, neither early nor late treatment with NAC had an effect on IVNV or retinal avascularity. Although apocynin had no effect on IVNV, it reduced both avascular retina (p=0.017) and retinal cleaved caspase-3 determined by western blot (p=0.021). In cryosections from OIR eyes, cleaved caspase-3 positive cells co-labeled with some lectin-stained vessels, NG2 labeled cells, and with GFAP positive cells in the inner nuclear layer. We found that the intravascular expression of gp91(phox) co-localized mostly with CD31 and some CD68 positive cells.
Conclusions: Our results do not support the antioxidant properties of NAC as effective in reducing IVNV or avascular retina in the 50/10 OIR rat model. Apocynin reduced avascularity and apoptosis in the OIR model perhaps through pathways triggered by ROS generation but upstream from LHP production. Further study and consideration may be given to apocynin or NAD(P)H oxidase inhibitors as adjunctive therapy for ROP to reduce the avascular retina.
Figures




References
-
- Katz ML, Robison WG., Jr Autoxidative damage to the retina: potential role in retinopathy of prematurity. Birth Defects Orig Artic Ser. 1988;24:237–48. - PubMed
-
- Penn JS. Oxygen-induced retinopathy in the rat: possible contribution of peroxidation reactions. Doc Ophthalmol. 1990;74:179–86. - PubMed
-
- Phelps DL, Rosenbaum AL. Vitamin E in kitten oxygen-induced retinopathy. II. Blockage of vitreal neovascularization. Arch Ophthalmol. 1979;97:1522–6. - PubMed
-
- Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci. 1993;34:576–85. - PubMed
-
- Rodieck W. The vertebrate retina; principles of structure and function. San Francisco: Freeman; 1973.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
