Lon protease degrades transfer-messenger RNA-tagged proteins
- PMID: 17616591
- PMCID: PMC2045158
- DOI: 10.1128/JB.00860-07
Lon protease degrades transfer-messenger RNA-tagged proteins
Abstract
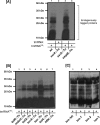
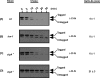
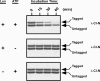
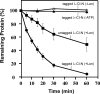
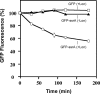
Bacterial trans translation is activated when translating ribosomes are unable to elongate or terminate properly. Small protein B (SmpB) and transfer-messenger RNA (tmRNA) are the two known factors required for and dedicated to trans translation. tmRNA, encoded by the ssrA gene, is a bifunctional molecule that acts both as a tRNA and as an mRNA during trans translation. The functions of tmRNA ensure that stalled ribosomes are rescued, the causative defective mRNAs are degraded, and the incomplete polypeptides are marked for targeted proteolysis. We present in vivo and in vitro evidence that demonstrates a direct role for the Lon ATP-dependent protease in the degradation of tmRNA-tagged proteins. In an endogenous protein tagging assay, lon mutants accumulated excessive levels of tmRNA-tagged proteins. In a reporter protein tagging assay with lambda-CI-N, the protein product of a nonstop mRNA construct designed to activate trans translation, lon mutant cells efficiently tagged the reporter protein, but the tagged protein exhibited increased stability. Similarly, a green fluorescent protein (GFP) construct containing a hard-coded C-terminal tmRNA tag (GFP-SsrA) exhibited increased stability in lon mutant cells. Most significantly, highly purified Lon preferentially degraded the tmRNA-tagged forms of proteins compared to the untagged forms. Based on these results, we conclude that Lon protease participates directly in the degradation of tmRNA-tagged proteins.
Figures

References
-
- Amerik, A., V. K. Antonov, A. E. Gorbalenya, S. A. Kotova, T. V. Rotanova, and E. V. Shimbarevich. 1991. Site-directed mutagenesis of La protease. A catalytically active serine residue. FEBS Lett. 287:211-214. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Stuhl (ed.). 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
-
- Barends, S., J. Wower, and B. Kraal. 2000. Kinetic parameters for tmRNA binding to alanyl-tRNA synthetase and elongation factor Tu from Escherichia coli. Biochemistry 39:2652-2658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

