The las enzymes control pyruvate metabolism in Lactococcus lactis during growth on maltose
- PMID: 17616595
- PMCID: PMC2045170
- DOI: 10.1128/JB.00902-07
The las enzymes control pyruvate metabolism in Lactococcus lactis during growth on maltose
Abstract
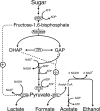
The fermentation pattern of Lactococcus lactis with altered activities of the las enzymes was examined on maltose. The wild type converted 65% of the maltose to mixed acids. An increase in phosphofructokinase or lactate dehydrogenase expression shifted the fermentation towards homolactic fermentation, and with a high level of expression of the las operon the fermentation was homolactic.
Figures


Similar articles
-
Control analysis as a tool to understand the formation of the las operon in Lactococcus lactis.FEBS J. 2005 May;272(9):2292-303. doi: 10.1111/j.1742-4658.2005.04656.x. FEBS J. 2005. PMID: 15853813
-
Transcriptional activation of the glycolytic las operon and catabolite repression of the gal operon in Lactococcus lactis are mediated by the catabolite control protein CcpA.Mol Microbiol. 1998 Nov;30(4):789-98. doi: 10.1046/j.1365-2958.1998.01111.x. Mol Microbiol. 1998. PMID: 10094627
-
Twofold reduction of phosphofructokinase activity in Lactococcus lactis results in strong decreases in growth rate and in glycolytic flux.J Bacteriol. 2001 Jun;183(11):3458-67. doi: 10.1128/JB.183.11.3458-3467.2001. J Bacteriol. 2001. PMID: 11344154 Free PMC article.
-
Physiology of pyruvate metabolism in Lactococcus lactis.Antonie Van Leeuwenhoek. 1996 Oct;70(2-4):253-67. doi: 10.1007/BF00395936. Antonie Van Leeuwenhoek. 1996. PMID: 8879410 Review.
-
Experimental determination of control of glycolysis in Lactococcus lactis.Antonie Van Leeuwenhoek. 2002 Aug;82(1-4):237-48. Antonie Van Leeuwenhoek. 2002. PMID: 12369190 Review.
Cited by
-
Metabolic control analysis: a tool for designing strategies to manipulate metabolic pathways.J Biomed Biotechnol. 2008;2008:597913. doi: 10.1155/2008/597913. J Biomed Biotechnol. 2008. PMID: 18629230 Free PMC article. Review.
-
Molecular and metabolic adaptations of Lactococcus lactis at near-zero growth rates.Appl Environ Microbiol. 2015 Jan;81(1):320-31. doi: 10.1128/AEM.02484-14. Epub 2014 Oct 24. Appl Environ Microbiol. 2015. PMID: 25344239 Free PMC article.
-
Intelligent host engineering for metabolic flux optimisation in biotechnology.Biochem J. 2021 Oct 29;478(20):3685-3721. doi: 10.1042/BCJ20210535. Biochem J. 2021. PMID: 34673920 Free PMC article. Review.
-
Escherichia coli robustly expresses ATP synthase at growth rate-maximizing concentrations.FEBS J. 2022 Aug;289(16):4925-4934. doi: 10.1111/febs.16401. Epub 2022 Mar 17. FEBS J. 2022. PMID: 35175666 Free PMC article.
-
Monte-Carlo modeling of the central carbon metabolism of Lactococcus lactis: insights into metabolic regulation.PLoS One. 2014 Sep 30;9(9):e106453. doi: 10.1371/journal.pone.0106453. eCollection 2014. PLoS One. 2014. PMID: 25268481 Free PMC article.
References
-
- Andersen, H. W., M. B. Pedersen, K. Hammer, and P. R. Jensen. 2001. Lactate dehydrogenase has no control on lactate production but has a strong negative control on formate production in Lactococcus lactis. Eur. J. Biochem. 268:6379-6389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

