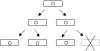
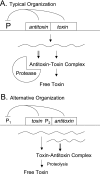
Hypothetical functions of toxin-antitoxin systems
- PMID: 17616596
- PMCID: PMC1951896
- DOI: 10.1128/JB.00958-07
Hypothetical functions of toxin-antitoxin systems
Figures

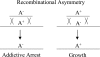
Comment on
-
What is the benefit to Escherichia coli of having multiple toxin-antitoxin systems in its genome?J Bacteriol. 2007 Sep;189(17):6101-8. doi: 10.1128/JB.00527-07. Epub 2007 May 18. J Bacteriol. 2007. PMID: 17513477 Free PMC article.
References
-
- Aguirre-Ramirez, M., J. Ramirez-Santos, L. Van Melderen, and M. C. Gomez-Eichelmann. 2006. Expression of the F plasmid ccd toxin-antitoxin system in Escherichia coli cells under nutritional stress. Can. J. Microbiol. 52:24-30. - PubMed
-
- Arcus, V. L., K. Backbro, A. Roos, E. L. Daniel, and E. N. Baker. 2004. Distant structural homology leads to the functional characterization of an archaeal PIN domain as an exonuclease. J. Biol. Chem. 279:16471-16478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

