Structural characterization of the ribosome maturation protein, RimM
- PMID: 17616598
- PMCID: PMC1951897
- DOI: 10.1128/JB.00024-07
Structural characterization of the ribosome maturation protein, RimM
Abstract
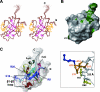
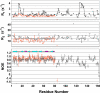
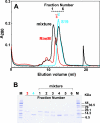
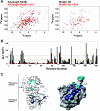
The RimM protein has been implicated in the maturation of the 30S ribosomal subunit. It binds to ribosomal protein S19, located in the head domain of the 30S subunit. Multiple sequence alignments predicted that RimM possesses two domains in its N- and C-terminal regions. In the present study, we have produced Thermus thermophilus RimM in both the full-length form (162 residues) and its N-terminal fragment, spanning residues 1 to 85, as soluble proteins in Escherichia coli and have performed structural analyses by nuclear magnetic resonance spectroscopy. Residues 1 to 80 of the RimM protein fold into a single structural domain adopting a six-stranded beta-barrel fold. On the other hand, the C-terminal region of RimM (residues 81 to 162) is partly folded in solution. Analyses of 1H-15N heteronuclear single quantum correlation spectra revealed that a wide range of residues in the C-terminal region, as well as the residues in the vicinity of a hydrophobic patch in the N-terminal domain, were dramatically affected upon complex formation with ribosomal protein S19.
Figures


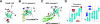

Similar articles
-
The PRC-barrel domain of the ribosome maturation protein RimM mediates binding to ribosomal protein S19 in the 30S ribosomal subunits.RNA. 2004 Nov;10(11):1798-812. doi: 10.1261/rna.7720204. RNA. 2004. PMID: 15496525 Free PMC article.
-
Preliminary NMR studies of Thermus thermophilus ribosomal protein S19 overproduced in Escherichia coli.FEBS Lett. 1997 Sep 29;415(2):155-9. doi: 10.1016/s0014-5793(97)01112-5. FEBS Lett. 1997. PMID: 9350986
-
The ribosomal protein S8 from Thermus thermophilus VK1. Sequencing of the gene, overexpression of the protein in Escherichia coli and interaction with rRNA.Eur J Biochem. 1994 Jul 15;223(2):437-45. doi: 10.1111/j.1432-1033.1994.tb19011.x. Eur J Biochem. 1994. PMID: 7519982
-
Structural Basis for the C-Terminal Domain of Mycobacterium tuberculosis Ribosome Maturation Factor RimM to Bind Ribosomal Protein S19.Biomolecules. 2021 Apr 18;11(4):597. doi: 10.3390/biom11040597. Biomolecules. 2021. PMID: 33919647 Free PMC article.
-
OB-fold: growing bigger with functional consistency.Curr Protein Pept Sci. 2003 Jun;4(3):195-206. doi: 10.2174/1389203033487207. Curr Protein Pept Sci. 2003. PMID: 12769718 Review.
Cited by
-
Escherichia coli rimM and yjeQ null strains accumulate immature 30S subunits of similar structure and protein complement.RNA. 2013 Jun;19(6):789-802. doi: 10.1261/rna.037523.112. Epub 2013 Apr 23. RNA. 2013. PMID: 23611982 Free PMC article.
-
Deconstructing ribosome construction.Trends Biochem Sci. 2009 May;34(5):256-63. doi: 10.1016/j.tibs.2009.01.011. Epub 2009 Apr 17. Trends Biochem Sci. 2009. PMID: 19376708 Free PMC article. Review.
-
Dissecting the in vivo assembly of the 30S ribosomal subunit reveals the role of RimM and general features of the assembly process.Nucleic Acids Res. 2013 Feb 1;41(4):2609-20. doi: 10.1093/nar/gks1256. Epub 2013 Jan 4. Nucleic Acids Res. 2013. PMID: 23293003 Free PMC article.
-
Protein Assistants of Small Ribosomal Subunit Biogenesis in Bacteria.Microorganisms. 2022 Mar 30;10(4):747. doi: 10.3390/microorganisms10040747. Microorganisms. 2022. PMID: 35456798 Free PMC article. Review.
-
The effect of ribosome assembly cofactors on in vitro 30S subunit reconstitution.J Mol Biol. 2010 Apr 23;398(1):1-7. doi: 10.1016/j.jmb.2010.02.036. Epub 2010 Feb 24. J Mol Biol. 2010. PMID: 20188109 Free PMC article.
References
-
- Alix, J. H. 1993. Extrinsic factors in ribosome assembly, p. 173-184. In K. H. Nierhaus, F. Franceschi, and A. R. Subramanian (ed.), The translational apparatus: structure, function, regulation, evolution. Plenum, New York, NY.
-
- Allen, G. S., A. Zavialov, R. Gursky, M. Ehrenberg, and J. Frank. 2005. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell 121:703-712. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

