Purification and three-dimensional electron microscopy structure of the Neisseria meningitidis type IV pilus biogenesis protein PilG
- PMID: 17616599
- PMCID: PMC1951934
- DOI: 10.1128/JB.00648-07
Purification and three-dimensional electron microscopy structure of the Neisseria meningitidis type IV pilus biogenesis protein PilG
Abstract
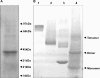
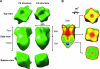
Type IV pili are surface-exposed retractable fibers which play a key role in the pathogenesis of Neisseria meningitidis and other gram-negative pathogens. PilG is an integral inner membrane protein and a component of the type IV pilus biogenesis system. It is related by sequence to the extensive GspF family of secretory proteins, which are involved in type II secretion processes. PilG was overexpressed and purified from Escherichia coli membranes by detergent extraction and metal ion affinity chromatography. Analysis of the purified protein by perfluoro-octanoic acid polyacrylamide gel electrophoresis showed that PilG formed dimers and tetramers. A three-dimensional (3-D) electron microscopy structure of the PilG multimer was determined using single-particle averaging applied to samples visualized by negative staining. Symmetry analysis of the unsymmetrized 3-D volume provided further evidence that the PilG multimer is a tetramer. The reconstruction also revealed an asymmetric bilobed structure approximately 125 A in length and 80 A in width. The larger lobe within the structure was identified as the N terminus by location of Ni-nitrilotriacetic acid nanogold particles to the N-terminal polyhistidine tag. We propose that the smaller lobe corresponds to the periplasmic domain of the protein, with the narrower "waist" region being the transmembrane section. This constitutes the first report of a 3-D structure of a member of the GspF family and suggests a physical basis for the role of the protein in linking cytoplasmic and periplasmic protein components of the type II secretion and type IV pilus biogenesis systems.
Figures




References
-
- Branden, C. I., and T. A. Jones. 1990. Between objectivity and subjectivity. Nature 343:687-689.
-
- Burrows, L. L. 2005. Weapons of mass retraction. Mol. Microbiol. 57:878-888. - PubMed
-
- Carbonnelle, E., S. Helaine, X. Nassif, and V. Pelicic. 2006. A systematic genetic analysis in Neisseria meningitidis defines the Pil proteins required for assembly, functionality, stabilization and export of type IV pili. Mol. Microbiol. 61:1510-1522. - PubMed
-
- Carbonnelle, E., S. Helaine, L. Prouvensier, X. Nassif, and V. Pelicic. 2005. Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occurring after pilus assembly, essential for fibre stability and function. Mol. Microbiol. 55:54-64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

