G-protein control of the ribosome-associated stress response protein SpoT
- PMID: 17616600
- PMCID: PMC1951942
- DOI: 10.1128/JB.00315-07
G-protein control of the ribosome-associated stress response protein SpoT
Abstract
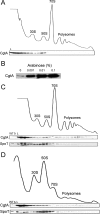
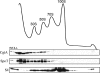
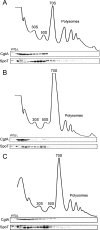
The bacterial response to stress is controlled by two proteins, RelA and SpoT. RelA generates the alarmone (p)ppGpp under amino acid starvation, whereas SpoT is responsible for (p)ppGpp hydrolysis and for synthesis of (p)ppGpp under a variety of cellular stress conditions. It is widely accepted that RelA is associated with translating ribosomes. The cellular location of SpoT, however, has been controversial. SpoT physically interacts with the ribosome-associated GTPase CgtA, and we show here that, under an optimized salt condition, SpoT is also associated with a pre-50S particle. Analysis of spoT and cgtA mutants and strains overexpressing CgtA suggests that the ribosome associations of SpoT and CgtA are mutually independent. The steady-state level of (p)ppGpp is increased in a cgtA mutant, but the accumulation of (p)ppGpp during amino acid starvation is not affected, providing strong evidence that CgtA regulates the (p)ppGpp level during exponential growth but not during the stringent response. We show that CgtA is not associated with pre-50S particles during amino acid starvation, indicating that under these conditions in which (p)ppGpp accumulates, CgtA is not bound either to the pre-50S particle or to SpoT. We propose that, in addition to its role as a 50S assembly factor, CgtA promotes SpoT (p)ppGpp degradation activity on the ribosome and that the loss of CgtA from the ribosome is necessary for maximal (p)ppGpp accumulation under stress conditions. Intriguingly, we found that in the absence of spoT and relA, cgtA is still an essential gene in Escherichia coli.
Figures

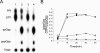
References
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Buglino, J., V. Shen, P. Hakimian, and C. D. Lima. 2002. Structural and biochemical analysis of the Obg GTP binding protein. Structure 10:1581-1592. - PubMed
-
- Butland, G., J. M. Peregrin-Alvarez, J. Li, W. Yang, X. Yang, V. Canadien, A. Starostine, D. Richards, B. Beattie, N. Krogan, M. Davey, J. Parkinson, J. Greenblatt, and A. Emili. 2005. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433:531-537. - PubMed
-
- Cashel, M., D. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1488-1496. In F. C. Neidhardt, R. Curtis, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

