Control of DegP-dependent degradation of c-type cytochromes by heme and the cytochrome c maturation system in Escherichia coli
- PMID: 17616605
- PMCID: PMC1951939
- DOI: 10.1128/JB.00656-07
Control of DegP-dependent degradation of c-type cytochromes by heme and the cytochrome c maturation system in Escherichia coli
Abstract
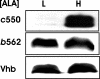
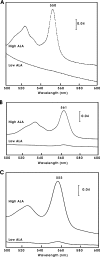
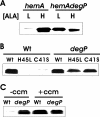
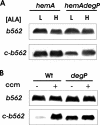
c-Type cytochromes are located partially or completely in the periplasm of gram-negative bacteria, and the heme prosthetic group is covalently bound to the protein. The cytochrome c maturation (Ccm) multiprotein system is required for transport of heme to the periplasm and its covalent linkage to the peptide. Other cytochromes and hemoglobins contain a noncovalently bound heme and do not require accessory proteins for assembly. Here we show that Bradyrhizobium japonicum cytochrome c550 polypeptide accumulation in Escherichia coli was heme dependent, with very low levels found in heme-deficient cells. However, apoproteins of the periplasmic E. coli cytochrome b562 or the cytosolic Vitreoscilla hemoglobin (Vhb) accumulated independently of the heme status. Mutation of the heme-binding cysteines of cytochrome c550 or the absence of Ccm also resulted in a low apoprotein level. These levels were restored in a degP mutant strain, showing that apocytochrome c550 is degraded by the periplasmic protease DegP. Introduction of the cytochrome c heme-binding motif CXXCH into cytochrome b562 (c-b562) resulted in a c-type cytochrome covalently bound to heme in a Ccm-dependent manner. This variant polypeptide was stable in heme-deficient cells but was degraded by DegP in the absence of Ccm. Furthermore, a Vhb variant containing a periplasmic signal peptide and a CXXCH motif did not form a c-type cytochrome, but accumulation was Ccm dependent nonetheless. The data show that the cytochrome c heme-binding motif is an instability element and that stabilization by Ccm does not require ligation of the heme moiety to the protein.
Figures

Similar articles
-
A cytochrome b562 variant with a c-type cytochrome CXXCH heme-binding motif as a probe of the Escherichia coli cytochrome c maturation system.J Biol Chem. 2003 Dec 26;278(52):52075-83. doi: 10.1074/jbc.M307196200. Epub 2003 Oct 7. J Biol Chem. 2003. PMID: 14534316
-
The histidine of the c-type cytochrome CXXCH haem-binding motif is essential for haem attachment by the Escherichia coli cytochrome c maturation (Ccm) apparatus.Biochem J. 2005 Jul 15;389(Pt 2):587-92. doi: 10.1042/BJ20041894. Biochem J. 2005. PMID: 15801911 Free PMC article.
-
The Escherichia coli cytochrome c maturation (Ccm) system does not detectably attach heme to single cysteine variants of an apocytochrome c.J Biol Chem. 2002 Sep 13;277(37):33559-63. doi: 10.1074/jbc.M204963200. Epub 2002 Jun 4. J Biol Chem. 2002. PMID: 12048216
-
What is the substrate specificity of the System I cytochrome c biogenesis apparatus?Biochem Soc Trans. 2006 Feb;34(Pt 1):150-1. doi: 10.1042/BST0340150. Biochem Soc Trans. 2006. PMID: 16417507 Review.
-
Periplasmic quality control in biogenesis of outer membrane proteins.Biochem Soc Trans. 2015 Apr;43(2):133-8. doi: 10.1042/BST20140217. Biochem Soc Trans. 2015. PMID: 25849907 Review.
Cited by
-
Domain swapping oligomerization of thermostable c-type cytochrome in E. coli cells.Sci Rep. 2016 Feb 3;6:19334. doi: 10.1038/srep19334. Sci Rep. 2016. PMID: 26838805 Free PMC article.
-
Cytochrome c biogenesis: the Ccm system.Trends Microbiol. 2010 Jun;18(6):266-74. doi: 10.1016/j.tim.2010.03.006. Epub 2010 Apr 8. Trends Microbiol. 2010. PMID: 20382024 Free PMC article. Review.
-
Compensatory thio-redox interactions between DsbA, CcdA and CcmG unveil the apocytochrome c holdase role of CcmG during cytochrome c maturation.Mol Microbiol. 2008 Nov;70(3):652-66. doi: 10.1111/j.1365-2958.2008.06441.x. Epub 2008 Sep 10. Mol Microbiol. 2008. PMID: 18786143 Free PMC article.
-
NapB Restores cytochrome c biosynthesis in bacterial dsbD-deficient mutants.Commun Biol. 2022 Jan 21;5(1):87. doi: 10.1038/s42003-022-03034-3. Commun Biol. 2022. PMID: 35064202 Free PMC article.
-
The thioreduction component CcmG confers efficiency and the heme ligation component CcmH ensures stereo-specificity during cytochrome c maturation.J Biol Chem. 2017 Aug 11;292(32):13154-13167. doi: 10.1074/jbc.M117.794586. Epub 2017 Jun 20. J Biol Chem. 2017. PMID: 28634234 Free PMC article.
References
-
- Allen, J. W., P. D. Barker, and S. J. Ferguson. 2003. A cytochrome b562 variant with a c-type cytochrome CXXCH heme-binding motif as a probe of the Escherichia coli cytochrome c maturation system. J. Biol. Chem. 278:52075-52083. - PubMed
-
- Arslan, E., H. Schulz, R. Zufferey, P. Kunzler, and L. Thöny-Meyer. 1998. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 251:744-747. - PubMed
-
- Barker, P. D., E. P. Nerou, S. M. Freund, and I. M. Fearnley. 1995. Conversion of cytochrome b562 to c-type cytochromes. Biochemistry 34:15191-15203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

