Retinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cells
- PMID: 17616676
- PMCID: PMC4133940
- DOI: 10.1158/0008-5472.CAN-06-4424
Retinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cells
Abstract
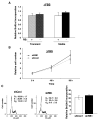
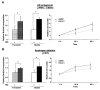
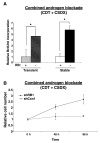
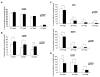
The retinoblastoma tumor suppressor protein (RB), a critical mediator of cell cycle progression, is functionally inactivated in the majority of human cancers, including prostatic adenocarcinoma. The importance of RB tumor suppressor function in this disease is evident because 25% to 50% of prostatic adenocarcinomas harbor aberrations in RB pathway. However, no previous studies challenged the consequence of RB inactivation on tumor cell proliferation or therapeutic response. Here, we show that RB depletion facilitates deregulation of specific E2F target genes, but does not confer a significant proliferative advantage in the presence of androgen. However, RB-deficient cells failed to elicit a cytostatic response (compared with RB proficient isogenic controls) when challenged with androgen ablation, AR antagonist, or combined androgen blockade. These data indicate that RB deficiency can facilitate bypass of first-line hormonal therapies used to treat prostate cancer. Given the established effect of RB on DNA damage checkpoints, these studies were then extended to determine the impact of RB depletion on the response to cytotoxic agents used to treat advanced disease. In this context, RB-deficient prostate cancer cells showed enhanced susceptibility to cell death induced by only a selected subset of cytotoxic agents (antimicrotubule agents and a topoisomerase inhibitor). Combined, these data indicate that RB depletion dramatically alters the cellular response to therapeutic intervention in prostate cancer cells and suggest that RB status could potentially be developed as a marker for effectively directing therapy.
Figures

References
-
- Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Kolvenbag GJ, Nash A. Bicalutamide dosages used in the treatment of prostate cancer. Prostate. 1999;39:47–53. - PubMed
-
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. - PubMed
-
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. - PubMed
-
- Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate. 2004;61:332–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

