Lymphocyte gene expression in subjects fed a low-choline diet differs between those who develop organ dysfunction and those who do not
- PMID: 17616785
- PMCID: PMC2587282
- DOI: 10.1093/ajcn/86.1.230
Lymphocyte gene expression in subjects fed a low-choline diet differs between those who develop organ dysfunction and those who do not
Abstract
Background: Some humans fed a low-choline diet develop hepatosteatosis, liver and muscle damage, and lymphocyte apoptosis. The risk of developing such organ dysfunction is increased by the presence of single-nucleotide polymorphisms (SNPs) in genes involved in folate and choline metabolism.
Objective: We investigated whether these changes that occur in the expression of many genes when humans are fed a low-choline diet differ between subjects who develop organ dysfunction and those who do not. We also investigated whether expression changes were dependent on the presence of the SNPs of interest.
Design: Thirty-three subjects aged 20-67 y were fed for 10 d a baseline diet containing the recommended adequate intake of choline. They then were fed a low-choline diet for up to 42 d or until they developed organ dysfunction. Blood was collected at the end of each phase, and peripheral lymphocytes were isolated and used for genotyping and for gene expression profiling with the use of microarray hybridization.
Results: Feeding a low-choline diet changed the expression of 259 genes, and the profiles of subjects who developed and those who did not develop signs of organ dysfunction differed. Group clustering and gene ontology analyses found that the diet-induced changes in gene expression profiles were significantly influenced by the SNPs of interest and that the gene expression phenotype of the variant gene carriers differed significantly even with the baseline diet.
Conclusion: These findings support our hypothesis that a person's susceptibility to organ dysfunction when fed a low-choline diet is modulated by specific SNPs in genes involved in folate and choline metabolism.
Figures
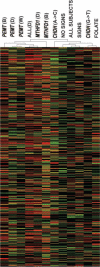

 , no change; □, underexpression within each comparison group (by significance analysis by microarray; the false discovery rate was <5%) as indicated in Table 1. ALL SUBJECTS, comparison of all subjects (the end of the depletion phrase compared with the end of the baseline phase); SIGNS, the subjects who developed organ dysfunction when fed the low-choline diet; NO SIGNS, the subjects who did not develop organ dysfunction (comparing the end of the depletion phase with the end of the baseline phase). For each polymorphism, the gene symbol is indicated. The choline dehydrogenase single-nucleotide polymorphisms are indicated by the nucleotide replacement, and the sample is at the end of depletion phase. The notation “(B)” after the gene symbol indicates the sample after the baseline (adequate choline) phase; “(D)” after the gene symbol indicates the sample after the low-choline diet phase; “(W)” after the gene symbol indicates women only after the depletion phase. FOLATE compares subjects with and without folate supplementation at the end of the depletion phase. The number of arrays for each comparison is provided in Table 1.
, no change; □, underexpression within each comparison group (by significance analysis by microarray; the false discovery rate was <5%) as indicated in Table 1. ALL SUBJECTS, comparison of all subjects (the end of the depletion phrase compared with the end of the baseline phase); SIGNS, the subjects who developed organ dysfunction when fed the low-choline diet; NO SIGNS, the subjects who did not develop organ dysfunction (comparing the end of the depletion phase with the end of the baseline phase). For each polymorphism, the gene symbol is indicated. The choline dehydrogenase single-nucleotide polymorphisms are indicated by the nucleotide replacement, and the sample is at the end of depletion phase. The notation “(B)” after the gene symbol indicates the sample after the baseline (adequate choline) phase; “(D)” after the gene symbol indicates the sample after the low-choline diet phase; “(W)” after the gene symbol indicates women only after the depletion phase. FOLATE compares subjects with and without folate supplementation at the end of the depletion phase. The number of arrays for each comparison is provided in Table 1.Similar articles
-
Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans.Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):16025-30. doi: 10.1073/pnas.0504285102. Epub 2005 Oct 18. Proc Natl Acad Sci U S A. 2005. PMID: 16236726 Free PMC article.
-
Choline deficiency increases lymphocyte apoptosis and DNA damage in humans.Am J Clin Nutr. 2006 Jul;84(1):88-94. doi: 10.1093/ajcn/84.1.88. Am J Clin Nutr. 2006. PMID: 16825685 Free PMC article. Clinical Trial.
-
Common genetic polymorphisms affect the human requirement for the nutrient choline.FASEB J. 2006 Jul;20(9):1336-44. doi: 10.1096/fj.06-5734com. FASEB J. 2006. PMID: 16816108 Free PMC article.
-
Nutritional genomics: defining the dietary requirement and effects of choline.J Nutr. 2011 Mar;141(3):531-4. doi: 10.3945/jn.110.130369. Epub 2011 Jan 26. J Nutr. 2011. PMID: 21270363 Free PMC article. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Choline and contribution to normal liver function of the foetus and exclusively breastfed infants: evaluation of a health claim pursuant to Article 14 of Regulation (EC) No 1924/2006.EFSA J. 2023 Jul 26;21(7):e08115. doi: 10.2903/j.efsa.2023.8115. eCollection 2023 Jul. EFSA J. 2023. PMID: 37502017 Free PMC article.
-
Metabolomics as a Promising Resource Identifying Potential Biomarkers for Inflammatory Bowel Disease.J Clin Med. 2021 Feb 6;10(4):622. doi: 10.3390/jcm10040622. J Clin Med. 2021. PMID: 33562024 Free PMC article. Review.
-
Choline in immunity: a key regulator of immune cell activation and function.Front Immunol. 2025 Aug 1;16:1617077. doi: 10.3389/fimmu.2025.1617077. eCollection 2025. Front Immunol. 2025. PMID: 40821837 Free PMC article. Review.
-
De novo fatty acid synthesis at the mitotic exit is required to complete cellular division.Cell Cycle. 2014;13(5):859-68. doi: 10.4161/cc.27767. Epub 2014 Jan 13. Cell Cycle. 2014. PMID: 24418822 Free PMC article.
-
MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming d9-choline.Am J Clin Nutr. 2011 Feb;93(2):348-55. doi: 10.3945/ajcn.110.005975. Epub 2010 Dec 1. Am J Clin Nutr. 2011. PMID: 21123458 Free PMC article. Clinical Trial.
References
-
- Institute of Medicine, National Academy of Sciences . Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. National Academy Press; Washington, DC: 1998. Choline. pp. 390–422. - PubMed
-
- Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–9. - PubMed
-
- Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

