Analysis of the contribution of the globin and reductase domains to the ligand-binding properties of bacterial haemoglobins
- PMID: 17617059
- PMCID: PMC2267399
- DOI: 10.1042/BJ20070668
Analysis of the contribution of the globin and reductase domains to the ligand-binding properties of bacterial haemoglobins
Abstract
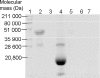
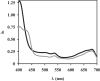
Bacterial Hbs (haemoglobins), like VHb (Vitreoscilla sp. Hb), and flavoHbs (flavohaemoglobins), such as FHP (Ralstonia eutropha flavoHb), have different autoxidation and ligand-binding rates. To determine the influence of each domain of flavoHbs on ligand binding, we have studied the kinetic ligand-binding properties of oxygen, carbon monoxide and nitric oxide to the chimaeric proteins, FHPg (truncated form of FHP comprising the globin domain alone) and VHb-Red (fusion protein between VHb and the C-terminal reductase domain of FHP) and compared them with those of their natural counterparts, FHP and VHb. Moreover, we also analysed polarity and solvent accessibility to the haem pocket of these proteins. The rate constants for the engineered proteins, VHb-Red and FHPg, do not differ significantly from those of their natural counterparts, VHb and FHP respectively. Our results suggest that the globin domain structure controls the reactivity towards oxygen, carbon monoxide and nitric oxide. The presence or absence of a reductase domain does not affect the affinity to these ligands.
Figures


Similar articles
-
Expression of Alcaligenes eutrophus flavohemoprotein and engineered Vitreoscilla hemoglobin-reductase fusion protein for improved hypoxic growth of Escherichia coli.Appl Environ Microbiol. 2000 Jan;66(1):98-104. doi: 10.1128/AEM.66.1.98-104.2000. Appl Environ Microbiol. 2000. PMID: 10618209 Free PMC article.
-
Chimeric Vitreoscilla hemoglobin (VHb) carrying a flavoreductase domain relieves nitrosative stress in Escherichia coli: new insight into the functional role of VHb.Appl Environ Microbiol. 2002 Jan;68(1):152-60. doi: 10.1128/AEM.68.1.152-160.2002. Appl Environ Microbiol. 2002. PMID: 11772621 Free PMC article.
-
Interaction of Vitreoscilla hemoglobin with membrane lipids.Biochemistry. 2006 Apr 4;45(13):4069-76. doi: 10.1021/bi052277n. Biochemistry. 2006. PMID: 16566580
-
Haemoglobins of Mycobacteria: structural features and biological functions.Adv Microb Physiol. 2013;63:147-94. doi: 10.1016/B978-0-12-407693-8.00005-4. Adv Microb Physiol. 2013. PMID: 24054797 Review.
-
Bacterial hemoglobins and flavohemoglobins: versatile proteins and their impact on microbiology and biotechnology.FEMS Microbiol Rev. 2003 Oct;27(4):525-45. doi: 10.1016/S0168-6445(03)00056-1. FEMS Microbiol Rev. 2003. PMID: 14550944 Review.
Cited by
-
Heterologous expression of flavohemoglobin in Rhodotorula toruloides led to improved lipid production.Biotechnol Lett. 2025 May 26;47(3):58. doi: 10.1007/s10529-025-03597-1. Biotechnol Lett. 2025. PMID: 40418250
References
-
- Wittenberg J. B., Bolognesi M., Wittenberg B. A., Guertin M. Truncated hemoglobins a new family of hemoglobins widely distributed in bacteria, unicellular eukaryotes and plants. J. Biol. Chem. 2002;277:871–874. - PubMed
-
- Tarricone C., Galizzi A., Coda A., Ascenzi P., Bolognesi M. Unusual structure of the oxygen-binding site in the dimeric bacterial hemoglobin from Vitreoscilla sp. Structure. 1997;5:497–507. - PubMed
-
- Ilari A., Bonamore A., Farina A., Johnson K. A., Boffi A. The X-ray structure of ferric Escherichia coli flavohemoglobin reveals an unexpected geometry of the distal heme pocket. J. Biol. Chem. 2002;277:23725–23732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

