Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake
- PMID: 17617060
- PMCID: PMC2267400
- DOI: 10.1042/BJ20070705
Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake
Abstract
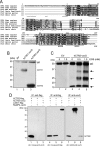
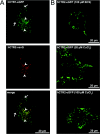
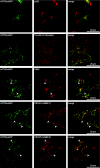
High-affinity cellular copper uptake is mediated by the CTR (copper transporter) 1 family of proteins. The highly homologous hCTR (human CTR) 2 protein has been identified, but its function in copper uptake is currently unknown. To characterize the role of hCTR2 in copper homoeostasis, epitope-tagged hCTR2 was transiently expressed in different cell lines. hCTR2-vsvG (vesicular-stomatitis-virus glycoprotein) predominantly migrated as a 17 kDa protein after imunoblot analysis, consistent with its predicted molecular mass. Chemical cross-linking resulted in the detection of higher-molecular-mass complexes containing hCTR2-vsvG. Furthermore, hCTR2-vsvG was co-immunoprecipitated with hCTR2-FLAG, suggesting that hCTR2 can form multimers, like hCTR1. Transiently transfected hCTR2-eGFP (enhanced green fluorescent protein) was localized exclusively to late endosomes and lysosomes, and was not detected at the plasma membrane. To functionally address the role of hCTR2 in copper metabolism, a novel transcription-based copper sensor was developed. This MRE (metal-responsive element)-luciferase reporter contained four MREs from the mouse metallothionein 1A promoter upstream of the firefly luciferase open reading frame. Thus the MRE-luciferase reporter measured bioavailable cytosolic copper. Expression of hCTR1 resulted in strong activation of the reporter, with maximal induction at 1 muM CuCl2, consistent with the K(m) of hCTR1. Interestingly, expression of hCTR2 significantly induced MRE-luciferase reporter activation in a copper-dependent manner at 40 and 100 microM CuCl2. Taken together, these results identify hCTR2 as an oligomeric membrane protein localized in lysosomes, which stimulates copper delivery to the cytosol of human cells at relatively high copper concentrations. This work suggests a role for endosomal and lysosomal copper pools in the maintenance of cellular copper homoeostasis.
Figures



Similar articles
-
Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells.Biochem J. 2008 Feb 1;409(3):731-40. doi: 10.1042/BJ20071025. Biochem J. 2008. PMID: 17944601
-
Cu(II) Binding to the N-Terminal Model Peptide of the Human Ctr2 Transporter at Lysosomal and Extracellular pH.Inorg Chem. 2019 Jun 3;58(11):7488-7498. doi: 10.1021/acs.inorgchem.9b00711. Epub 2019 May 14. Inorg Chem. 2019. PMID: 31083932
-
Divalent metal transporter 1 in the kidney proximal tubule is expressed in late endosomes/lysosomal membranes: implications for renal handling of protein-metal complexes.Am J Physiol Renal Physiol. 2006 Jun;290(6):F1525-33. doi: 10.1152/ajprenal.00359.2005. Epub 2006 Jan 31. Am J Physiol Renal Physiol. 2006. PMID: 16449358
-
Posttranslational regulation of copper transporters.J Biol Inorg Chem. 2010 Jan;15(1):37-46. doi: 10.1007/s00775-009-0592-7. Epub 2009 Oct 8. J Biol Inorg Chem. 2010. PMID: 19813030 Review.
-
A structural perspective on copper uptake in eukaryotes.Biometals. 2007 Jun;20(3-4):705-16. doi: 10.1007/s10534-006-9054-7. Epub 2007 Jan 9. Biometals. 2007. PMID: 17211682 Review.
Cited by
-
SLC31 (CTR) family of copper transporters in health and disease.Mol Aspects Med. 2013 Apr-Jun;34(2-3):561-70. doi: 10.1016/j.mam.2012.07.011. Mol Aspects Med. 2013. PMID: 23506889 Free PMC article. Review.
-
Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper.Biosci Rep. 2013 Jul 16;33(4):e00049. doi: 10.1042/BSR20130014. Biosci Rep. 2013. PMID: 23738776 Free PMC article. Review.
-
Cuproptosis: Unraveling the Mechanisms of Copper-Induced Cell Death and Its Implication in Cancer Therapy.Cancers (Basel). 2024 Feb 2;16(3):647. doi: 10.3390/cancers16030647. Cancers (Basel). 2024. PMID: 38339398 Free PMC article. Review.
-
Copper homeostasis and the ubiquitin proteasome system.Metallomics. 2023 Mar 6;15(3):mfad010. doi: 10.1093/mtomcs/mfad010. Metallomics. 2023. PMID: 36822629 Free PMC article. Review.
-
Membrane transporters as mediators of Cisplatin effects and side effects.Scientifica (Cairo). 2012;2012:473829. doi: 10.6064/2012/473829. Epub 2012 Nov 25. Scientifica (Cairo). 2012. PMID: 24278698 Free PMC article. Review.
References
-
- Culotta V. C., Lin S. J., Schmidt P., Klomp L. W., Casareno R. L., Gitlin J. Intracellular pathways of copper trafficking in yeast and humans. Adv. Exp. Med. Biol. 1999;448:247–254. - PubMed
-
- de Bie P., van de Sluis B., Klomp L., Wijmenga C. The many faces of the copper metabolism protein MURR1/COMMD1. J. Hered. 2005;96:803–811. - PubMed
-
- Himelblau E., Amasino R. M. Delivering copper within plant cells. Curr. Opin. Plant Biol. 2000;3:205–210. - PubMed
-
- Rae T. D., Schmidt P. J., Pufahl R. A., Culotta V. C., O'Halloran T. V. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. - PubMed
-
- Casareno R. L., Waggoner D., Gitlin J. D. The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J. Biol. Chem. 1998;273:23625–23628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

