Dofetilide-induced long QT and torsades de pointes
- PMID: 17617063
- PMCID: PMC6932735
- DOI: 10.1111/j.1542-474X.2007.00161.x
Dofetilide-induced long QT and torsades de pointes
Abstract
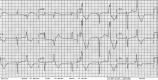
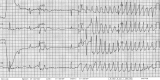
Dofetilide, a new class III antiarrhythmic agent, has been approved as an antiarrhythmic agent for the treatment of atrial fibrillation and atrial flutter. Dofetilide selectively inhibits the rapid component of the delayed rectifier potassium current resulting in a prolongation of the effective refractory period. Like other drugs that affect potassium currents, the prolonged QT interval occurring in the patients treated with dofetilide can be complicated by torsades de pointes. We report four cases of dofetilide-induced QT prolongation and torsades de pointes. We discuss the risk factors for the development of dofetilide-induced long QT and torsades de pointes and review the current literature.
Figures
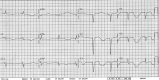
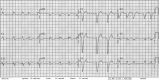
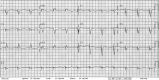
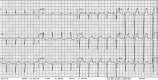
References
-
- Mounsey JP, DiMarco JP. Cardiovascular drugs. Dofetilide. Circulation 2000;102:2665–2670. - PubMed
-
- Falk RH, Decara JM. Dofetilide: A new pure class III antiarrhythmic agent. Am Heart J 2000;140:697–706. - PubMed
-
- Rousseau MF, Massart PE, Van Eyll C, et al Cardiac and hemodynamic effects of intravenous dofetilide in patients with heart failure. Am J Cardiol 2001;87:1250–1254. - PubMed
-
- Singh S, Zoble RG, Yellen L, et al Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: The symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE‐D) study. Circulation 2000;102:2385–2390. - PubMed
-
- Guanzon AV, Crouch MA. Phase IV trial evaluating the effectiveness and safety of dofetilide. Ann Pharmacother 2004;38(7–8):1142–1147. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

