Alterations in striatal dopamine catabolism precede loss of substantia nigra neurons in a mouse model of juvenile neuronal ceroid lipofuscinosis
- PMID: 17617387
- PMCID: PMC4790084
- DOI: 10.1016/j.brainres.2007.05.018
Alterations in striatal dopamine catabolism precede loss of substantia nigra neurons in a mouse model of juvenile neuronal ceroid lipofuscinosis
Abstract
Batten disease, or juvenile neuronal ceroid lipofuscinosis (JNCL), results from mutations in the CLN3 gene. This disorder presents clinically around the age of 5 years with visual deficits progressing to include seizures, cognitive impairment, motor deterioration, hallucinations, and premature death by the third to fourth decade of life. The motor deficits include coordination and gait abnormalities, myoclonic jerks, inability to initiate movements, and spasticity. Previous work from our laboratory has identified an early reduction in catechol-O-methyltransferase (COMT), an enzyme responsible for the efficient degradation of dopamine. Alterations in the kinetics of dopamine metabolism could cause the accumulation of undegraded or unsequestered dopamine leading to the formation of toxic dopamine intermediates. We report an imbalance in the catabolism of dopamine in 3 month Cln3(-/-) mice persisting through 9 months of age that may be causal to oxidative damage within the striatum at 9 months of age. Combined with the previously reported inflammatory changes and loss of post-synaptic D1alpha receptors, this could facilitate cell loss in striatal projection regions and underlie a general locomotion deficit that becomes apparent at 12 months of age in Cln3(-/-) mice. This study provides evidence for early changes in the kinetics of COMT in the Cln3(-/-) mouse striatum, affecting the turnover of dopamine, likely leading to neuron loss and motor deficits. These data provide novel insights into the basis of motor deficits in JNCL and how alterations in dopamine catabolism may result in oxidative damage and localized neuronal loss in this disorder.
Figures

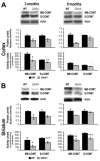
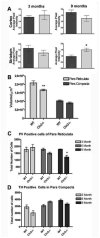



Decrease in both COMT levels and activity
Decrease in the metabolites dopamine and DOPAC
Decrease in the postsynaptic D1α receptor
No difference in the expression of DAT, MAO-B, VMAT, or NET
Similar articles
-
Cerebellar defects in a mouse model of juvenile neuronal ceroid lipofuscinosis.Brain Res. 2009 Apr 17;1266:93-107. doi: 10.1016/j.brainres.2009.02.009. Epub 2009 Feb 20. Brain Res. 2009. PMID: 19230832 Free PMC article.
-
Self-Complementary AAV9 Gene Delivery Partially Corrects Pathology Associated with Juvenile Neuronal Ceroid Lipofuscinosis (CLN3).J Neurosci. 2016 Sep 14;36(37):9669-82. doi: 10.1523/JNEUROSCI.1635-16.2016. J Neurosci. 2016. PMID: 27629717 Free PMC article.
-
Phenotypic characterization of a mouse model of juvenile neuronal ceroid lipofuscinosis.Neurobiol Dis. 2008 Feb;29(2):242-53. doi: 10.1016/j.nbd.2007.08.017. Epub 2007 Sep 7. Neurobiol Dis. 2008. PMID: 17962032 Free PMC article.
-
Juvenile neuronal ceroid-lipofuscinosis (Batten disease): a brief review and update.Curr Mol Med. 2007 Sep;7(6):603-8. doi: 10.2174/156652407781695729. Curr Mol Med. 2007. PMID: 17896996 Review.
-
Vision loss in juvenile neuronal ceroid lipofuscinosis (CLN3 disease).Ann N Y Acad Sci. 2016 May;1371(1):55-67. doi: 10.1111/nyas.12990. Epub 2016 Jan 8. Ann N Y Acad Sci. 2016. PMID: 26748992 Free PMC article. Review.
Cited by
-
Moving towards effective therapeutic strategies for Neuronal Ceroid Lipofuscinosis.Orphanet J Rare Dis. 2016 Apr 16;11:40. doi: 10.1186/s13023-016-0414-2. Orphanet J Rare Dis. 2016. PMID: 27083890 Free PMC article. Review.
-
A novel interaction of CLN3 with nonmuscle myosin-IIB and defects in cell motility of Cln3(-/-) cells.Exp Cell Res. 2011 Jan 1;317(1):51-69. doi: 10.1016/j.yexcr.2010.09.007. Epub 2010 Sep 17. Exp Cell Res. 2011. PMID: 20850431 Free PMC article.
-
A Case-controlled Investigation of Pain Experience and Sensory Function in Neuronal Ceroid Lipofuscinosis.Clin J Pain. 2015 Nov;31(11):998-1003. doi: 10.1097/AJP.0000000000000192. Clin J Pain. 2015. PMID: 25569218 Free PMC article.
-
Neuropeptide changes and neuroactive amino acids in CSF from humans and sheep with neuronal ceroid lipofuscinoses (NCLs, Batten disease).Neurochem Int. 2009 Dec;55(8):783-8. doi: 10.1016/j.neuint.2009.07.012. Epub 2009 Aug 5. Neurochem Int. 2009. PMID: 19664668 Free PMC article.
-
Finding the most appropriate mouse model of juvenile CLN3 (Batten) disease for therapeutic studies: the importance of genetic background and gender.Dis Model Mech. 2015 Apr;8(4):351-61. doi: 10.1242/dmm.018804. Dis Model Mech. 2015. PMID: 26035843 Free PMC article.
References
-
- Aberg L, Liewendahl K, Nikkinen P, Autti T, Rinne JO, Santavuori P. Decreased striatal dopamine transporter density in JNCL patients with parkinsonian symptoms. Neurology. 2000;54:1069–1074. - PubMed
-
- Aberg LE, Rinne JO, Rajantie I, Santavuori P. A favorable response to antiparkinsonian treatment in juvenile neuronal ceroid lipofuscinosis. Neurology. 2001;56:1236–1239. - PubMed
-
- Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–705. - PubMed
-
- Barisic N, Logan P, Pikija S, Skarpa D, Blau N. R208× mutation in CLN2 gene associated with reduced cerebrospinal fluid pterins in a girl with classic late infantile neuronal ceroid lipofuscinosis. Croat Med J. 2003;44:489–493. - PubMed
-
- Benedict JW, Sommers CA, Pearce DA. Progressive oxidative damage in the the CNS of a Murine Model of Juvenile Batten Disease. Journal of Neuroscience Research. 2007 In press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

