Two stereoisomers of spheroidene in the Rhodobacter sphaeroides R26 reaction center: a DFT analysis of resonance Raman spectra
- PMID: 17617552
- PMCID: PMC1913164
- DOI: 10.1529/biophysj.106.103473
Two stereoisomers of spheroidene in the Rhodobacter sphaeroides R26 reaction center: a DFT analysis of resonance Raman spectra
Abstract
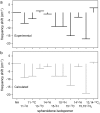
From a theoretical analysis of the resonance Raman spectra of 19 isotopomers of spheroidene reconstituted into the reaction center (RC) of Rhodobacter sphaeroides R26, we conclude that the carotenoid in the RC occurs in two configurations. The normal mode underlying the resonance Raman transition at 1239 cm(-1), characteristic for spheroidene in the RC, has been identified and found to uniquely refer to the cis nature of the 15,15' carbon-carbon double bond. Detailed analysis of the isotope-induced shifts of transitions in the 1500-1550 cm(-1) region proves that, besides the 15,15'-cis configuration, spheroidene in the RC adopts another cis-configuration, most likely the 13,14-cis configuration.
Figures




References
-
- Cogdell, R. J., and H. A. Frank. 1987. How carotenoids function in photosynthetic bacteria. Biochim. Biophys. Acta. 895:63–79. - PubMed
-
- Koyama, Y. 1991. Structures and functions of carotenoids in photosynthetic systems. J. Photochem. Photobiol. B Biol. 9:265–280.
-
- Cogdell, R. J., W. W. Parson, and M. A. Kerr. 1976. The type, amount, location, and energy transfer properties of the carotenoid in reaction centers from Rhodopseudomonas sphaeroides. Biochim. Biophys. Acta. 430:83–93. - PubMed
-
- Foote, C. S., Y. C. Chang, and R. W. Denny. 1970. Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. J. Am. Chem. Soc. 92:5216–5218. - PubMed
-
- Lutz, M., J. Kleo, and F. Reiss-Husson. 1976. Resonance Raman-scattering of bacteriochlorophyll, bacteriopheophytin and spheroidene in reaction centers of Rhodopseudomonas spheroides. Biochem. Biophys. Res. Commun. 69:711–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

