Low-dose parenteral busulfan provides an extended window for the infusion of hematopoietic stem cells in murine hosts
- PMID: 17618036
- PMCID: PMC2676902
- DOI: 10.1016/j.exphem.2007.05.009
Low-dose parenteral busulfan provides an extended window for the infusion of hematopoietic stem cells in murine hosts
Abstract
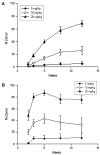
Objective: Myeloablative total body irradiation (TBI) in the setting of autologous transplantation of genetically modified hematopoietic stem cells (HSC) is associated with substantial toxicity. Nonmyeloablative doses of TBI are less toxic, but result in low-level engraftment of genetically modified HSCs. As an alternative to TBI, escalating doses of parenteral busulfan were tested for their hematologic toxicity, their ability to promote donor leukocyte engraftment, and the time window for such engraftment.
Materials and methods: Hematologic toxicity of busulfan was assessed in C57BL6 mice after single nonmyeloablative doses of intraperitoneal busulfan ranging from 1 to 40 mg/kg by serial complete blood counts monitored up to 40 days. The level of donor engraftment attainable after nonmyeloablative busulfan was determined by infusion of 20 million congenic murine bone marrow nucleated cells (BMNC) following 5 to 40 mg/kg of busulfan. To determine the effects of delayed HSC infusions, BMNCs were infused 1, 10, 15, and 20 days after a single dose of 10 mg/kg of busulfan.
Results: Busulfan doses from 1 to 40 mg/kg produced hematologic toxicity that was most pronounced in the 2nd to 3rd week. In transplantation experiments, dose-dependent donor leukocyte engraftment was attained with levels >70% after only 20 mg/kg of busulfan. Similar levels of engraftment were achieved even when infusion of BMNCs was delayed up to 20 days after busulfan injection.
Conclusion: Nonmyeloablative parenteral busulfan produced transient myelosuppressive effects, clinically relevant levels of engraftment, and an extended time window for HSC infusion in murine hosts.
Figures



Similar articles
-
Lymphoid reconstitution after transplantation of congenic hematopoietic cells in busulfan-treated mice.Blood. 1991 Dec 15;78(12):3312-6. Blood. 1991. PMID: 1683798
-
Combining Mobilizing Agents with Busulfan to Reduce Chemotherapy-Based Conditioning for Hematopoietic Stem Cell Transplantation.Cells. 2021 Apr 30;10(5):1077. doi: 10.3390/cells10051077. Cells. 2021. PMID: 33946560 Free PMC article.
-
Busulfan pharmacokinetics, toxicity, and low-dose conditioning for autologous transplantation of genetically modified hematopoietic stem cells in the rhesus macaque model.Exp Hematol. 2006 Feb;34(2):132-9. doi: 10.1016/j.exphem.2005.10.010. Exp Hematol. 2006. PMID: 16459181 Free PMC article.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Antibody based conditioning for allogeneic hematopoietic stem cell transplantation.Front Immunol. 2022 Oct 20;13:1031334. doi: 10.3389/fimmu.2022.1031334. eCollection 2022. Front Immunol. 2022. PMID: 36341432 Free PMC article. Review.
Cited by
-
Reinterpretation of evidence advanced for neo-oogenesis in mammals, in terms of a finite oocyte reserve.J Ovarian Res. 2011 Jan 6;4(1):1. doi: 10.1186/1757-2215-4-1. J Ovarian Res. 2011. PMID: 21211009 Free PMC article.
-
Testicular weight deficits, altered variables of antioxidant defense system, spermatogenesis impairment, and inflammation induced by busulfan injection are ameliorated by gallic acid administration in rats.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):6765-6779. doi: 10.1007/s00210-024-03699-z. Epub 2024 Dec 14. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39673638
-
Wild-type macrophages reverse disease in heme oxygenase 1-deficient mice.Blood. 2014 Aug 28;124(9):1522-30. doi: 10.1182/blood-2014-02-554162. Epub 2014 Jun 24. Blood. 2014. PMID: 24963040 Free PMC article.
-
Generation of NOD SCID mice with near-complete deletions of Il2rg and Prkdc for human cancer and HSC engraftment.Transgenic Res. 2025 Jul 11;34(1):35. doi: 10.1007/s11248-025-00454-9. Transgenic Res. 2025. PMID: 40643795 Free PMC article.
-
Total body irradiation must be delivered at high dose for efficient engraftment and tolerance in a rhesus stem cell gene therapy model.Mol Ther Methods Clin Dev. 2016 Sep 14;3:16059. doi: 10.1038/mtm.2016.59. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 27652288 Free PMC article.
References
-
- Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. - PubMed
-
- Woolfrey AE, Pulsipher MA, Storb R. Non-myeloablative hematopoietic cell transplant for treatment of nonmalignant disorders in children. Int J Hematol. 2002;76(Suppl 2):271–277. - PubMed
-
- Storb RF, Lucarelli G, McSweeney PA, Childs RW. Hematopoietic cell transplantation for benign hematological disorders and solid tumors. Hematology Am Soc Hematol Educ Prog. 2003:372. - PubMed
-
- Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. - PubMed
-
- Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

