Symptom management for cancer patients: a trial comparing two multimodal interventions
- PMID: 17618080
- PMCID: PMC2043403
- DOI: 10.1016/j.jpainsymman.2006.11.018
Symptom management for cancer patients: a trial comparing two multimodal interventions
Abstract
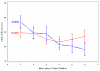
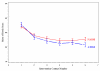
The results of a randomized controlled trial that tested the effects of eight-week, six-contact multidimensional interactive interventions for symptom management are presented. Four hundred and thirty-five cancer patients with solid tumors undergoing chemotherapy were randomized to receive either nurse-assisted symptom management (NASM) or automated telephone symptom management (ATSM). A prior trial established the effectiveness of NASM compared with conventional care. Seventeen symptoms commonly experienced by patients undergoing chemotherapy were rated on a scale from 0 to 10 and were evaluated at baseline, at each of the six intervention contacts, and postintervention observation at 10 weeks. Both groups achieved significant reduction in symptom severity over baseline, and there was no difference between groups on symptom severity at 10 weeks. Randomization accounted for possible reductions in severity due to response shifts. Severity of symptoms reported by patients at each of the six intervention contacts was measured using a Rasch model. Symptom pattern was different for lung and non-lung cancer patients, and they were analyzed separately. Longitudinal analyses revealed that lung cancer patients with greater symptom severity withdrew from later intervention contacts of the ATSM. The results suggest that both NASM and ATSM achieved a clinically significant reduction in symptom severity. The NASM may be more effective than ATSM in retaining lung cancer patients in the intervention. Further testing of ATSM supplemented by NASM for patients with severe symptoms is warranted.
Figures





References
-
- Kroenke K. Studying symptoms: sampling and measurement issues. Ann Intern Med. 2001;134:844–853. - PubMed
-
- Cleeland C, Mendoza T, Wang X, et al. Assessing symptom distress in cancer patients. Cancer. 2000;89:634–1646. - PubMed
-
- Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. - PubMed
-
- Bruera E, Kuehn N, Miller M, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliative Care. 1991;7:6–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

