A functional link between area MSTd and heading perception based on vestibular signals
- PMID: 17618278
- PMCID: PMC2430983
- DOI: 10.1038/nn1935
A functional link between area MSTd and heading perception based on vestibular signals
Abstract
Recent findings of vestibular responses in part of the visual cortex--the dorsal medial superior temporal area (MSTd)--indicate that vestibular signals might contribute to cortical processes that mediate the perception of self-motion. We tested this hypothesis in monkeys trained to perform a fine heading discrimination task solely on the basis of inertial motion cues. The sensitivity of the neuronal responses was typically lower than that of psychophysical performance, and only the most sensitive neurons rivaled behavioral performance. Responses recorded in MSTd were significantly correlated with perceptual decisions, and the correlations were strongest for the most sensitive neurons. These results support a functional link between MSTd and heading perception based on inertial motion cues. These cues seem mainly to be of vestibular origin, as labyrinthectomy produced a marked elevation of psychophysical thresholds and abolished MSTd responses. This study provides evidence that links single-unit activity to spatial perception mediated by vestibular signals, and supports the idea that the role of MSTd in self-motion perception extends beyond optic flow processing.
Figures


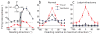




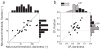
References
-
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971;34:661–675. - PubMed
-
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol. 1976;39:985–995. - PubMed
-
- Angelaki DE, Hess BJ. Self-motion-induced eye movements: effects on visual acuity and navigation. Nat Rev Neurosci. 2005;6:966–976. - PubMed
-
- Raphan T, Cohen B. The vestibulo-ocular reflex in three dimensions. Exp Brain Res. 2002;145:1–27. - PubMed
-
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res. 1976;26:59–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

