Relationship between petal abscission and programmed cell death in Prunus yedoensis and Delphinium belladonna
- PMID: 17618454
- PMCID: PMC2039816
- DOI: 10.1007/s00425-007-0566-3
Relationship between petal abscission and programmed cell death in Prunus yedoensis and Delphinium belladonna
Abstract
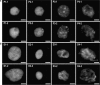
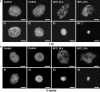
Depending on the species, the end of flower life span is characterized by petal wilting or by abscission of petals that are still fully turgid. Wilting at the end of petal life is due to programmed cell death (PCD). It is not known whether the abscission of turgid petals is preceded by PCD. We studied some parameters that indicate PCD: chromatin condensation, a decrease in nuclear diameter, DNA fragmentation, and DNA content per nucleus, using Prunus yedoensis and Delphinium belladonna which both show abscission of turgid petals at the end of floral life. No DNA degradation, no chromatin condensation, and no change in nuclear volume was observed in P. yedoensis petals, prior to abscission. In abscising D. belladonna petals, in contrast, considerable DNA degradation was found, chromatin was condensed and the nuclear volume considerably reduced. Following abscission, the nuclear area in both species drastically increased, and the chromatin became unevenly distributed. Similar chromatin changes were observed after dehydration (24 h at 60 degrees C) of petals severed at the time of flower opening, and in dehydrated petals of Ipomoea nil and Petunia hybrida, severed at the time of flower opening. In these flowers the petal life span is terminated by wilting rather than abscission. It is concluded that the abscission of turgid petals in D. belladonna was preceded by a number of PCD indicators, whereas no such evidence for PCD was found at the time of P. yedoensis petal abscission. Dehydration of the petal cells, after abscission, was associated with a remarkable nuclear morphology which was also found in younger petals subjected to dehydration. This nuclear morphology has apparently not been described previously, for any organism.
Figures






References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s004250000381', 'is_inner': False, 'url': 'https://doi.org/10.1007/s004250000381'}, {'type': 'PubMed', 'value': '11216841', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11216841/'}]}
- Gunawardena AHLAN, Pearce DM, Jackson MB, Hawes CR, Evans DE (2001) Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212:205–214 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0014-5793(97)00133-6', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0014-5793(97)00133-6'}, {'type': 'PubMed', 'value': '9119078', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9119078/'}]}
- Orzáez D, Granell A (1997) The plant homologue of the defender against apoptotic death gene is down-regulated during senescence of flower petals. FEBS Lett 404:275–278 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1469-8137.2004.01226.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1469-8137.2004.01226.x'}, {'type': 'PubMed', 'value': '15720658', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15720658/'}]}
- Pak C, van Doorn WG (2005) Delay of Iris flower senescence by protease inhibitors. New Phytol 165:473–480 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1034/j.1399-3054.2003.1170213.x', 'is_inner': False, 'url': 'https://doi.org/10.1034/j.1399-3054.2003.1170213.x'}]}
- van der Kop DAM., Ruys G, Dees D, van der Schoot C, de Boer AD, van Doorn WG (2003) Expression of defender against apoptotic death (DAD-1) in Iris and Dianthus petals. Physiol Plant 117:256–263
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1006/anbo.2000.1357', 'is_inner': False, 'url': 'https://doi.org/10.1006/anbo.2000.1357'}]}
- van Doorn WG (2001) Categories of petal senescence and abscission: a re-evaluation. Ann Bot 87:447–456
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

