Remyelination of the injured spinal cord
- PMID: 17618995
- PMCID: PMC2605400
- DOI: 10.1016/S0079-6123(06)61030-3
Remyelination of the injured spinal cord
Abstract
Contusive spinal cord injury (SCI) can result in necrosis of the spinal cord, but often long white matter tracts outside of the central necrotic core are demyelinated. One experimental strategy to improve functional outcome following SCI is to transplant myelin-forming cells to remyelinate these axons and improve conduction. This review focuses on transplantation studies using olfactory ensheathing cell (OEC) to improve functional outcome in experimental models of SCI and demyelination. The biology of the OEC, and recent experimental research and clinical studies using OECs as a potential cell therapy candidate are discussed.
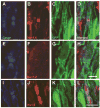
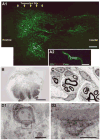
Figures






References
-
- Au E, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;41:224–236. - PubMed
-
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;10:269–277. - PubMed
-
- Barnett SC, Alexander CL, Iwashita Y, Gilson JM, Crowther J, Clark L, Dunn LT, Papanastassiou V, Kennedy PG, Franklin RJ. Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain. 2000;123:1581–1588. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Blakemore WF, Crang AJ. The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system. J Neurol Sci. 1985;70:207–223. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

