The vaccinia virus E8R gene product is required for formation of transcriptionally active virions
- PMID: 17619043
- PMCID: PMC2185540
- DOI: 10.1016/j.virol.2007.05.002
The vaccinia virus E8R gene product is required for formation of transcriptionally active virions
Abstract
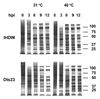
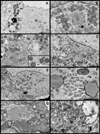
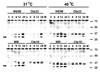
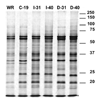
Two vaccinia virus temperature-sensitive mutants were mapped to the E8R gene and subjected to phenotypic characterization. Dts23 contains a missense mutation in the coding region of E8R (L81F), and in Cts19 the initiating methionine codon is changed from ATG to ATA (M1I). The two ts mutants display normal patterns of gene expression and DNA replication during infection. The E8 protein is synthesized exclusively late during infection and packaged into virion cores Western blot analysis revealed that E8 synthesis is reduced in Dts23 infected cells at permissive (31 degrees C) and non-permissive temperature (39.7 degrees C) and absent in Cts19 infection under both conditions. Dts23 virions produced at 39.7 degrees C were indistinguishable in appearance from wt virions. Cts19 fails to produce identifiable viral structures when incubated at 39.7 degrees C. Purified Dts23 virions produced at 39.7 degrees C contain reduced amounts of E8 and have a high particle to infectivity ratio; purified Cts19 virions grown at 31 degrees C also show reduced infectivity and do not contain detectable E8. Dts23 grown at 39.7 degrees C could enter cells but failed to synthesize early mRNA or produce CPE. Soluble extracts from mutant virions were active in a promoter dependent in vitro transcription assay, however intact mutant cores were defective in transcription. We suggest that E8 plays a subtle role in virion core structure that impacts directly or indirectly on core transcription.
Figures






References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York: John Wiley & Sons; 1994.
-
- Berger SL. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 2002;12:142–148. - PubMed
-
- Black EP, Moussatche N, Condit RC. Characterization of the interactions among vaccinia virus transcription factors G2R, A18R, and H5R. Virology. 1998;245:313–322. - PubMed
-
- Broyles SS. Vaccinia virus transcription. J. Gen. Virol. 2003;84:2293–2303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

