Studies of stimulus parameters for seizure disruption using neural network simulations
- PMID: 17619199
- PMCID: PMC2738632
- DOI: 10.1007/s00422-007-0166-0
Studies of stimulus parameters for seizure disruption using neural network simulations
Abstract
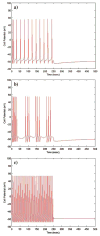
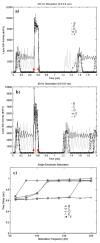
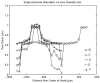
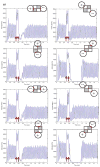
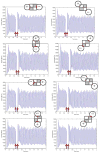
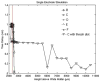
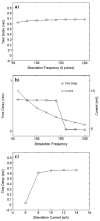
A large scale neural network simulation with realistic cortical architecture has been undertaken to investigate the effects of external electrical stimulation on the propagation and evolution of ongoing seizure activity. This is an effort to explore the parameter space of stimulation variables to uncover promising avenues of research for this therapeutic modality. The model consists of an approximately 800 mum x 800 mum region of simulated cortex, and includes seven neuron classes organized by cortical layer, inhibitory or excitatory properties, and electrophysiological characteristics. The cell dynamics are governed by a modified version of the Hodgkin-Huxley equations in single compartment format. Axonal connections are patterned after histological data and published models of local cortical wiring. Stimulation induced action potentials take place at the axon initial segments, according to threshold requirements on the applied electric field distribution. Stimulation induced action potentials in horizontal axonal branches are also separately simulated. The calculations are performed on a 16 node distributed 32-bit processor system. Clear differences in seizure evolution are presented for stimulated versus the undisturbed rhythmic activity. Data is provided for frequency dependent stimulation effects demonstrating a plateau effect of stimulation efficacy as the applied frequency is increased from 60 to 200 Hz. Timing of the stimulation with respect to the underlying rhythmic activity demonstrates a phase dependent sensitivity. Electrode height and position effects are also presented. Using a dipole stimulation electrode arrangement, clear orientation effects of the dipole with respect to the model connectivity is also demonstrated. A sensitivity analysis of these results as a function of the stimulation threshold is also provided.
Figures









References
-
-
Anderson WS. For original C codes of the neural network simulation, and for MPEGs of the network activity, the contact email is <email>wanderso@jhmi.edu</email>.
-
-
- Atkinson WJ, Young JH, Brezovich IA. An analytic solution for the potential due to a circular parallel plate capacitor. J Phys A: Math Gen. 1983;16:2837–2841.
-
- Av-Ron E. The role of a transient potassium current in a bursting neuron model. J Math Biol. 1994;33:71–87. - PubMed
-
- Av-Ron E, Parnas H, Segel LA. A basic biophysical model for bursting neurons. Biol Cybern. 1993;69:87–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

