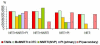Protease inhibitor associated mutations compromise the efficacy of therapy in human immunodeficiency virus-1 (HIV-1) infected pediatric patients: a cross-sectional study
- PMID: 17620130
- PMCID: PMC1959238
- DOI: 10.1186/1742-6405-4-15
Protease inhibitor associated mutations compromise the efficacy of therapy in human immunodeficiency virus-1 (HIV-1) infected pediatric patients: a cross-sectional study
Abstract
Background: Although the introduction of combined therapy with reverse transcriptase and protease inhibitors has resulted in considerable decrease in HIV related mortality; it has also induced the development of multiple drug-resistant HIV-1 variants. The few studies on HIV-1 mutagenesis in HIV infected children have not evaluated the impact of HIV-1 mutations on the clinical, virological and immunological presentation of HIV disease that is fundamental to optimizing the treatment regimens for these patients.
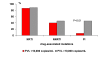
Results: A cross sectional study was conducted to evaluate the impact of treatment regimens and resistance mutation patterns on the clinical, virological, and immunological presentation of HIV disease in 41 children (25 male and 16 female) at the Robert Wood Johnson Pediatric AIDS Program in New Brunswick, New Jersey. The study participants were symptomatic and had preceding treatment history with combined ARV regimens including protease inhibitors (PIs), nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs). Fifteen (36.6%) children were treated with NRTI+NNRTI+ PI, 6 (14.6%) with NRTI+NNRTIs, 13 (31.7%) with NRTI+PIs, and the remaining 7 (17.1%) received NRTIs only. Combined ARV regimens did not significantly influence the incidence of NRTI and NNRTI associated mutations. The duration of ARV therapy and the child's age had no significant impact on the ARV related mutations. The clinico-immunological presentation of the HIV disease was not associated with ARV treatment regimens or number of resistance mutations. However, primary mutations in the protease (PR) gene increased the likelihood of plasma viral load (PVL) > or = 10,000 copies/mL irrespective of the child's age, duration of ARV therapy, presence of NRTI and NNRTI mutation. Viremia > or = 10,000 copies/mL was recorded in almost all the children with primary mutations in the PR region (n = 12/13, 92.3%) as compared with only 50.0% (n = 14/28) of HIV infected children without (PR-), P < 0.008. However, CD-4 T cells were not affected by the mutations in the PR gene of the HIV-1 isolates.
Conclusion: Primary PR resistance mutations significantly increase the likelihood for high viral replication in pediatric patients with moderate/severe HIV-1 infection, which may affect the long-term clinical prognosis of the HIV infected children.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous